Lipid Oxidation and Food Rancidity: Mechanisms, Analytical Methods, and Health Implications for Scientific Research
This article provides a comprehensive review of the chemical mechanisms of lipid oxidation and food rancidity, tailored for researchers, scientists, and drug development professionals.
Lipid Oxidation and Food Rancidity: Mechanisms, Analytical Methods, and Health Implications for Scientific Research
Abstract
This article provides a comprehensive review of the chemical mechanisms of lipid oxidation and food rancidity, tailored for researchers, scientists, and drug development professionals. It explores the foundational science of autoxidation, photo-oxidation, and hydrolytic rancidity, detailing the formation of primary and secondary oxidation products. The scope extends to advanced and traditional analytical methodologies for assessing lipid oxidation and antioxidant efficacy. Furthermore, the article examines strategies to control oxidation in complex food matrices and discusses the implications of lipid oxidation products on human health, including inflammation, carcinogenesis, and atherosclerosis, providing a critical link to biomedical research.
Deconstructing Rancidity: Fundamental Pathways and Chemical Mechanisms of Lipid Oxidation
Rancidity is a critical form of food spoilage involving the chemical degradation of fats and oils, leading to undesirable sensory properties and nutritional losses. Within the broader context of lipid oxidation and food rancidity research, understanding these degradation pathways is essential for developing effective preservation strategies across food and pharmaceutical industries. Rancidity occurs through three primary mechanisms: oxidative, hydrolytic, and microbial pathways [1] [2]. Each mechanism generates distinct breakdown compounds that contribute to off-flavors, off-odors, and potential health concerns.
This technical guide examines these rancidity pathways at a molecular level, providing researchers and drug development professionals with comprehensive mechanistic insights, analytical methodologies, and stabilization approaches. The complex interplay between these pathways significantly impacts product shelf life, nutritional quality, and safety, making their understanding crucial for both fundamental research and industrial applications.
Oxidative Rancidity
Mechanism of Oxidative Rancidity
Oxidative rancidity, primarily affecting unsaturated fatty acids, occurs via a free-radical chain reaction known as autoxidation when lipids encounter oxygen from air, light, or heat [3] [4]. This process unfolds in three distinct phases:
Initiation: Free radicals form as hydrogen atoms are abstracted from fatty acid chains, creating alkyl radicals (R•). This initiation is catalyzed by heat, light, or metal ions [5] [6]: RH + O₂ → R• + •OOH or RH → R• + •H
Propagation: Alkyl radicals rapidly react with atmospheric oxygen to form peroxyl radicals (ROO•), which subsequently abstract hydrogen from other fatty acids to form hydroperoxides (ROOH) and new alkyl radicals, propagating the chain reaction [3] [6]: R• + O₂ → ROO• ROO• + RH → ROOH + R•
Termination: The reaction cycle concludes when free radicals combine to form non-radical products [3] [6]: R• + R• → R-R ROO• + R• → ROOR
Hydroperoxides themselves are relatively tasteless and odorless, but their secondary decomposition yields volatile compounds—including aldehydes, ketones, alcohols, and hydrocarbons—responsible for the characteristic rancid odors and flavors [3] [4]. The rate of oxidation increases with the number of double bonds in fatty acids; thus, polyunsaturated fats are most vulnerable [7].
Factors Influencing Oxidative Rancidity
Multiple factors accelerate oxidative rancidity:
- Oxygen availability: Higher oxygen concentrations increase oxidation rates [1]
- Light exposure: Photo-oxidation occurs when light, especially UV, catalyzes radical formation [3]
- Temperature: Elevated temperatures exponentially accelerate oxidation rates [1]
- Metal catalysts: Trace amounts of iron, copper, and other transition metals catalyze initiation [1]
- Water activity: Moderate moisture levels can promote metal ion mobility and catalytic activity [1]
- Fatty acid composition: Higher unsaturated fat content increases susceptibility [1] [8]
Table 1: Primary Oxidation Products and Detection Methods
| Oxidation Stage | Key Compounds | Characteristic Effects | Detection Methods |
|---|---|---|---|
| Primary | Hydroperoxides, Peroxides | Minimal flavor impact, nutrient degradation | Peroxide Value (PV), Conjugated Dienes [5] [4] |
| Secondary | Aldehydes (hexanal, nonanal), Ketones, Alcohols | Rancid odors, off-flavors | p-Anisidine Value (p-AV), TBARS, Gas Chromatography [5] [7] |
| Tertiary | Polymers, Short-chain fatty acids | Texture changes, nutritional loss | Size-exclusion Chromatography, Viscosity measurements [5] |
Figure 1: Oxidative Rancidity Free Radical Chain Reaction
Hydrolytic Rancidity
Mechanism of Hydrolytic Rancidity
Hydrolytic rancidity involves the cleavage of ester bonds in triglycerides, releasing free fatty acids (FFAs) from the glycerol backbone [1] [9]. This process occurs through hydrolysis facilitated by either moisture, heat, or enzymatic activity:
Triglyceride + Water → Free Fatty Acids + Di/Monoglycerides + Glycerol
Short-chain free fatty acids (with ≤12 carbon atoms), particularly butyric acid (C4), caproic acid (C6), caprylic acid (C8), and capric acid (C10), are primarily responsible for the sharp, unpleasant, "goaty" odors associated with hydrolytic rancidity [1]. Butyric acid, for instance, contributes positively to flavor profiles in small quantities (as in cheese) but becomes offensive at higher concentrations—it is the primary odor compound in human vomit and used in stink bombs [1].
Enzymatic Hydrolysis
Lipases present naturally in foods or produced by microorganisms catalyze hydrolytic rancidity at ambient temperatures [9] [10]. In pearl millet, high lipase activity combined with substantial lipid content (~5-6%) accelerates hydrolytic rancidity, significantly limiting shelf-life despite its nutritional benefits [9]. Rice bran similarly contains active lipases that rapidly hydrolyze lipids upon milling, severely restricting its food applications [10].
Table 2: Short-Chain Free Fatty Acids in Hydrolytic Rancidity
| Free Fatty Acid | Carbon Atoms | Sensory Characteristics | Common Sources |
|---|---|---|---|
| Butyric acid | 4 | Rancid, cheesy, vomit-like | Butter, milk products [1] |
| Caproic acid | 6 | Pungent, sweaty | Goat milk, cheese [1] |
| Caprylic acid | 8 | Rancid, waxy | Coconut oil, palm oil [1] |
| Capric acid | 10 | Sour, unpleasant | Dairy fats, tropical oils [1] |
Figure 2: Hydrolytic Rancidity Pathway
Microbial Rancidity
Mechanism of Microbial Rancidity
Microbial rancidity results from microorganisms (bacteria, yeasts, molds) producing extracellular enzymes, particularly lipases and phospholipases, that hydrolyze fats into free fatty acids [2]. These liberated fatty acids can undergo further microbial transformation through β-oxidation, generating additional off-flavor compounds.
While microbial rancidity shares similarities with hydrolytic rancidity in its initial steps, the microbial origin introduces additional complexity. Different microbial species produce distinct enzymatic profiles, leading to diverse spoilage patterns. Furthermore, microbial activity is influenced by environmental factors including temperature, pH, water activity, and nutrient availability [2].
Context-Dependent Nature of Microbial Rancidity
The same enzymatic processes that cause spoilage in some foods are deliberately employed in cheese production to develop characteristic flavors [1]. For example, Penicillium roquefortii in Roquefort cheese and lipases from rennet paste in Fiore Sardo and Pecorino cheeses generate short-chain free fatty acids that create desirable spicy and peppery notes [1]. This demonstrates that rancidity is partly defined by cultural and sensory expectations rather than purely chemical processes.
Analytical Methods for Rancidity Assessment
Chemical and Instrumental Methods
Comprehensive rancidity assessment requires monitoring multiple indicators across different degradation stages:
- Peroxide Value (PV): Measures hydroperoxides (primary oxidation products) via iodometric titration; values <5 mEq/kg generally indicate fresh oils [5] [4]
- p-Anisidine Value (p-AV): Quantifies secondary oxidation products (aldehydes) through colorimetric reaction; higher values indicate advanced oxidation [5] [7]
- Thiobarbituric Acid Reactive Substances (TBARS): Detects malondialdehyde and related carbonyls as secondary oxidation markers; particularly useful for meat and low-fat products [5] [4]
- Free Fatty Acids (FFA): Measures hydrolytic rancidity via titration of liberated fatty acids [5]
- Gas Chromatography (GC): Identifies and quantifies specific volatile compounds responsible for off-flavors [5] [7]
- Oxidative Stability Index (OSI): Accelerates oxidation (typically at 60-110°C with air bubbling) to predict shelf-life and antioxidant efficacy [5]
Sensory Evaluation
Sensory analysis remains crucial despite advances in instrumental methods, as human perception ultimately defines acceptability [5] [2]. Trained panels evaluate visual characteristics, odors, and flavors using standardized scales. Sensory testing is particularly important for correlating chemical measurements with actual product quality.
Table 3: Standard Analytical Methods for Rancidity Assessment
| Method | Target Compounds | Application Scope | Advantages/Limitations |
|---|---|---|---|
| Peroxide Value (PV) | Hydroperoxides | Early-stage oxidation | Simple, sensitive to initial oxidation; Peroxides decompose over time [5] |
| p-Anisidine Value (p-AV) | Aldehydes, Ketones | Secondary oxidation | Specific to carbonyls; Limited for dark samples [5] [7] |
| TBARS | Malondialdehyde, Carbonyls | Meat, fish, low-fat foods | Sensitive for specific products; Not comprehensive for all volatiles [5] [4] |
| Free Fatty Acids (FFA) | Liberated fatty acids | Hydrolytic rancidity | Direct measure of hydrolysis; Requires factor adjustment for different fats [5] |
| Gas Chromatography | Volatile compounds | All food types | Identifies specific compounds; Expensive, requires expertise [5] [7] |
| Conjugated Dienes | Diene formation | Early oxidation of PUFAs | Rapid, inexpensive; Limited to early stages [4] |
Experimental Protocols
Protocol: Peroxide Value Determination
Principle: Peroxides and hydroperoxides in the sample oxidize iodide to iodine, which is quantified by titration with thiosulfate [5] [4].
Reagents:
- Solvent mixture: Glacial acetic acid/chloroform (3:2 v/v)
- Saturated potassium iodide (KI) solution
- Sodium thiosulfate (Na₂S₂O₃) titrant (0.01 N standardized)
- Starch indicator solution (1%)
Procedure:
- Accurately weigh 5.00 g of oil or fat sample into a 250 mL glass-stoppered flask
- Add 30 mL of acetic acid/chloroform solvent mixture and swirl to dissolve
- Add 0.5 mL of saturated KI solution, stopper, and swirl for 60 seconds
- Place in dark for exactly 5 minutes, then add 30 mL of distilled water
- Titrate with 0.01 N sodium thiosulfate until yellow color almost disappears
- Add 0.5 mL starch indicator and continue titration until blue color disappears
- Run blank determination simultaneously
Calculation: PV (meq O₂/kg) = [(S - B) × N × 1000] / sample weight (g) Where: S = sample titrant volume (mL), B = blank titrant volume (mL), N = thiosulfate normality
Protocol: Accelerated Storage Studies
Principle: The Schaal Oven Test accelerates oxidation by storing samples at elevated temperatures (typically 60°C) while monitoring oxidation indicators over time [7].
Procedure:
- Portion uniform samples (50 g) into open glass containers or permeable packaging
- Place in forced-air oven maintained at 60 ± 1°C
- Randomize container positions daily to ensure uniform heating
- Sample at predetermined intervals (0, 3, 6, 9, 12, 16, 20 days)
- Analyze samples for PV, p-AV, FFA, and/or specific volatiles via GC-MS
- Store sampled portions at -20°C in dark if not analyzed immediately
Data Interpretation: Plot oxidation parameters versus time to determine induction period and oxidation rate. Correlation with actual shelf-life requires validation studies.
Protocol: Lipase Activity Assay
Principle: Lipase activity is determined by measuring free fatty acids released from a triglyceride substrate under controlled conditions [9].
Reagents:
- Substrate: 1% tributyrin or olive oil emulsion in 50 mM Tris buffer (pH 7.0-8.0)
- Stopping solution: Acetone/ethanol (1:1)
- Titrant: 0.02 N NaOH with phenolphthalein indicator
Procedure:
- Incubate 1 mL enzyme extract with 5 mL substrate at 37°C for 30 minutes
- Stop reaction by adding 10 mL acetone/ethanol mixture
- Titrate immediately with 0.02 N NaOH to faint pink endpoint
- Run appropriate blank and control reactions
Calculation: One unit of lipase activity = 1 μmol FFA released per minute under assay conditions
Research Reagent Solutions
Table 4: Essential Research Reagents for Rancidity Studies
| Reagent/Chemical | Technical Function | Application Examples |
|---|---|---|
| tert-Butylhydroquinone (TBHQ) | Synthetic antioxidant, free radical scavenger | Fish oil stabilization, delaying oxidation initiation [7] [6] |
| Propyl Gallate (PG) | Synthetic phenolic antioxidant | Inhibits propagation phase in oils and fats [7] |
| Butylated Hydroxyanisole (BHA) | Antioxidant, donates hydrogen atoms | Preserves rendered products, functional in baking [1] [5] |
| Butylated Hydroxytoluene (BHT) | Chain-breaking antioxidant | Extends shelf-life of oils, animal feeds [1] [5] |
| Thiobarbituric Acid (TBA) | Reacts with malondialdehyde | TBARS assay for secondary oxidation products [5] [4] |
| p-Anisidine | Reacts with aldehydes | p-Anisidine value determination [5] [7] |
| Sodium Thiosulfate | Reductant for iodine titration | Peroxide value determination [5] [4] |
| Lipase from microbial sources | Hydrolyzes triglycerides | Model studies of enzymatic rancidity [9] |
Oxidative, hydrolytic, and microbial rancidity represent distinct but interconnected pathways of lipid degradation, each with characteristic mechanisms, compounds, and analytical approaches. Understanding these pathways at molecular level provides crucial insights for developing effective stabilization strategies across food and pharmaceutical industries. Contemporary research employs increasingly sophisticated methodologies including lipidomics and flavoromics to elucidate complex oxidation pathways and antioxidant mechanisms [7]. Future directions include developing natural antioxidant systems, optimizing delivery mechanisms, and establishing more accurate predictive models for shelf-life determination.
Autoxidation is a spontaneous, free-radical chain reaction between organic compounds and molecular oxygen at normal temperatures, which plays a critical role in the deterioration of fats and oils, leading to food rancidity [11]. This process represents a significant challenge in food science and nutrition research, as it directly impacts food quality, shelf-life, and sensory properties. The free radical-mediated mechanism of autoxidation is responsible for the gradual degradation of unsaturated lipids in various food matrices, generating off-flavors, unpleasant odors, and potentially toxic compounds that compromise nutritional value and consumer safety [9]. Understanding the fundamental principles of the free radical chain reaction—initiation, propagation, and termination—provides researchers with the theoretical foundation necessary to develop effective strategies for mitigating oxidative deterioration in food systems.
The particular susceptibility of polyunsaturated fatty acids (PUFAs) to autoxidation poses a substantial problem for nutrient-dense foods. For instance, pearl millet, recognized as a "nutricereal" due to its high nutritional value, contains 50-55% polyunsaturated fatty acids, primarily linoleic acid, which significantly contributes to its rapid quality deterioration post-milling [9]. This chemical instability necessitates comprehensive research into the mechanistic pathways of lipid oxidation to preserve the health benefits of such nutritionally superior foods. The study of autoxidation mechanisms thus represents an essential intersection of food chemistry, nutritional science, and material stability, with direct implications for food security, waste reduction, and public health.
The Free Radical Chain Reaction Mechanism
The autoxidation process follows a well-established free radical chain mechanism, originally characterized in rubber oxidation but universally applicable to lipid systems [11]. This mechanism consists of three distinct stages: initiation, propagation, and termination, each with characteristic reactions and radical species. The chain reaction nature of autoxidation explains its autocatalytic behavior, where the rate of oxidation accelerates progressively after an initial induction period, as reaction products themselves participate in generating additional radicals [12].
Initiation Phase
The initiation phase encompasses the initial generation of free radicals from non-radical precursor compounds. This step requires energy input, typically from heat, light, or metal catalysts, to break relatively weak chemical bonds [13] [11]. In lipid systems, initiation commonly occurs through the homolytic cleavage of hydroperoxides (ROOH), which may be present as trace impurities or formed through previous oxidation, or through direct hydrogen abstraction from unsaturated fatty acids by reactive oxygen species [14].
Table 1: Common Initiation Reactions in Lipid Autoxidation
| Initiator Type | Representative Reaction | Resulting Radicals | Comment |
|---|---|---|---|
| Hydroperoxides | ROOH → RO• + •OH | Alkoxy, hydroxyl | Thermal or photolytic cleavage |
| Metal Ions | ROOH + Mⁿ⁺ → RO• + M⁽ⁿ⁺¹⁾⁺ + OH⁻ | Alkoxy | Redox cycling |
| Singlet Oxygen | ¹O₂ + RH → ROO• | Peroxyl | Photooxygenation |
| Azo Compounds | R-N=N-R → 2R• + N₂ | Carbon-centered | Intentional initiator |
The initiation step is characterized by a net increase in the number of free radicals in the system, transitioning from zero radicals to two or more radical species [13] [12]. This phase typically demonstrates an induction period where little oxidative activity is observed, followed by progressively accelerating oxygen uptake as the reaction transitions to the propagation phase [11].
Propagation Phase
The propagation phase maintains the radical chain reaction through sequences where radicals react with non-radical substrates to generate new radical species, with no net change in the number of free radicals [13] [12]. This phase consists of two critical steps that cycle repeatedly, allowing a single initiation event to facilitate numerous oxidation cycles before termination.
The primary propagation sequence begins when a carbon-centered radical (R•) reacts rapidly with molecular oxygen to form a peroxyl radical (ROO•). The peroxyl radical then abstracts a hydrogen atom from another unsaturated lipid molecule (RH), generating a hydroperoxide (ROOH) and a new carbon-centered radical (R•) that continues the chain reaction [11]. The hydrogen abstraction step is rate-determining and depends significantly on the bond dissociation energy of the C-H bond involved, with allylic and bis-allylic hydrogens in polyunsaturated fatty acids being particularly susceptible to abstraction due to their relatively low bond dissociation energies [12].
Table 2: Key Propagation Reactions in Lipid Autoxidation
| Reaction Step | Chemical Equation | Radical Count (Reactants → Products) | Role in Chain Reaction |
|---|---|---|---|
| Oxygen addition | R• + O₂ → ROO• | 1 → 1 | Forms peroxyl radical |
| Hydrogen abstraction | ROO• + RH → ROOH + R• | 1 → 1 | Regroups chain carrier radical |
| Hydroperoxide decomposition | ROOH → RO• + •OH | 0 → 2 | Chain branching |
A single initiation event can propagate through thousands of these cycles before termination occurs, with estimates suggesting up to 10⁴ or more cycles per initiation event in some systems [13]. This amplification effect explains why even trace initiator concentrations can drive significant oxidative deterioration in lipid-containing foods.
Termination Phase
The termination phase encompasses reactions where two radical species combine to form non-radical products, resulting in a net decrease in the number of free radicals [13] [12]. These bimolecular reactions between radicals effectively halt the propagation cycle by removing radical species from the system.
Common termination reactions include combinations of peroxyl radicals (ROO•), alkoxyl radicals (RO•), and carbon-centered radicals (R•) to form stable, non-radical products such as alcohols, ketones, aldehydes, and dimeric or cross-linked species [11]. While termination reactions occur throughout the autoxidation process, they become increasingly significant as radical concentrations increase, eventually leading to reaction deceleration when radical generation can no longer keep pace with termination events.
The specific termination pathways operative in a given system depend on factors including radical mobility, concentration, and stability. In viscous or polymeric systems, termination may be limited by diffusion, leading to extended kinetic chains and more extensive oxidation. The cross-linking reactions during termination can lead to macromolecular aggregation and altered physical properties in oxidized lipids and food matrices [11].
Quantitative Analysis of Autoxidation Parameters
Understanding the kinetic and thermodynamic parameters of autoxidation provides researchers with predictive capabilities for lipid stability and enables targeted intervention strategies. Quantitative analysis of these parameters facilitates comparison between different lipid systems and assessment of antioxidant efficacy.
Table 3: Quantitative Parameters in Lipid Autoxidation
| Parameter | Typical Range | Significance | Analytical Methods |
|---|---|---|---|
| Bond Dissociation Energy (C-H) | ~80-100 kcal/mol | Determines H-abstraction susceptibility | Computational chemistry |
| Propagation Rate Constant (kₚ) | 10-10⁴ M⁻¹s⁻¹ | Measures chain carrying efficiency | Competitive kinetics |
| Oxygen uptake | Variable with unsaturation | Direct measure of oxidation extent | Oxygen electrode, manometry |
| Peroxide Value (PV) | 0->20 meq/kg | Early oxidation indicator | Titration, spectrophotometry |
| Induction period | Hours to months | Oxidative stability measure | Rancimat, OSI |
The rate of autoxidation depends significantly on the structure of the lipid substrate, with relative oxidation rates increasing exponentially with the degree of unsaturation. For example, the relative oxidation rates for fatty acids with 1, 2, 3, 4, 5, and 6 double bonds are approximately 1, 2, 3, 4, 5, and 6, respectively, though actual values vary depending on measurement conditions and specific molecular arrangements [11]. This structure-reactivity relationship explains why foods rich in polyunsaturated fats, such as pearl millet (containing 50-55% PUFAs), demonstrate particular susceptibility to rancidity development [9].
Experimental Protocols for Studying Autoxidation
Biomimetic Model Systems for Radical Reactions
Chemical biology approaches utilizing biomimetic models provide controlled environments for studying free radical processes under biologically relevant conditions. These systems enable detailed mechanistic studies without the complexity of intact biological matrices, facilitating the identification of reaction pathways and products [15].
Protocol: UV-Induced Isomerization of Cholesteryl Esters
- Sample Preparation: Dissolve cholesteryl esters (linoleate or arachidonate) in 2-propanol (15 mM concentration). Sonicicate for 15 minutes under argon to ensure complete solubilization.
- Radical Initiation: Transfer the solution to a quartz photochemical reactor. Add 2-mercaptoethanol in 2-propanol to reach 7 mM concentration (from a 2 M stock solution). Flush the reaction mixture with argon for 20 minutes to eliminate oxygen.
- UV Irradiation: Irradiate the reaction mixture using a 5.5W low-pressure mercury lamp at 22±2°C for 4 minutes. Monitor reaction progress by analytical silver-thin layer chromatography (Ag-TLC) to detect formation of mono-trans cholesteryl esters.
- Product Isolation: Quench the reaction at early stages to recover starting material for subsequent isomerization rounds. Purify mono-trans isomers of cholesteryl esters by preparative Ag-TLC using hexane-diethyl ether (9:1 v/v) as eluent for cholesteryl linoleate isomers, or hexane-diethyl ether-acetic acid (9:1:0.1 v/v) for cholesteryl arachidonate isomers [15].
This protocol generates reference compounds for detecting radical-mediated transformations in biological samples, enabling biomarker development for oxidation in complex food systems.
Accelerated Shelf-Life Testing for Food Rancidity
Accelerated storage studies simulate natural post-processing degradation under controlled conditions that promote oxidative reactions, enabling rapid assessment of rancidity development.
Protocol: Pearl Millet Flour Rancidity Induction
- Sample Preparation: Manually clean freshly harvested pearl millet grains to remove debris and non-viable seeds. Mill grains using a standardized grain mill under controlled conditions to ensure uniform flour particle size.
- Storage Conditions: Transfer freshly milled flour into translucent polypropylene bottles with perforated caps to permit controlled aeration. Store samples under ambient laboratory light conditions at constant temperature of 35±1°C to accelerate oxidative and hydrolytic deterioration.
- Sampling Time Points: Collect samples at designated intervals: M0 (immediately after milling, as fresh control), M1 (after 30 days storage, designated rancid flour), and M2 (after 60 days storage, designated severely rancid flour).
- Analysis: Subject samples to metabolomic profiling, enzymatic assays (lipase, lipoxygenase, peroxidase, polyphenol oxidase), and chemical rancidity indices (acid value, peroxide value, free fatty acid content) [9].
This protocol activates native enzymes and promotes oxidation of polyunsaturated fatty acids, effectively simulating flour storage conditions while enabling precise monitoring of rancidity progression.
Analytical Techniques for Oxidation Product Characterization
Comprehensive characterization of autoxidation products requires multidisciplinary analytical approaches spanning chromatographic, spectroscopic, and computational methods.
Protocol: Metabolomic Profiling of Oxidized Lipids
- Metabolite Extraction: Accurately weigh flour samples (50 mg) into pre-chilled microcentrifuge tubes. Spike with internal standard solution (10 μL containing 2-chlorophenylalanine and D₄-glutamic acid). Extract with 400 μL ice-cold 80% methanol in water.
- Homogenization: Vortex vigorously for 30 seconds followed by agitation using a bead mill homogenizer at 30 Hz for two cycles of 30 seconds, with 5-second rest intervals between cycles, maintaining samples on ice.
- Centrifugation and Extraction: Centrifuge at 14,000 × g for 10 minutes at 4°C. Transfer supernatant (polar extract). To remaining pellet, add 400 μL ice-cold chloroform, repeat vortexing, agitation, and centrifugation.
- LC-MS Analysis: Reconstitute dried extracts in acetonitrile:isopropanol:water (65:30:5, v/v/v) containing 0.1% formic acid. Perform metabolome profiling using UHPLC system coupled to Orbitrap mass spectrometer with C18 column separation and gradient elution with 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B) [9].
This protocol enables identification and quantification of a wide range of lipid oxidation products, facilitating development of molecular libraries for biomarker discovery in rancid food products.
Research Reagent Solutions for Autoxidation Studies
Table 4: Essential Research Reagents for Autoxidation Studies
| Reagent/Chemical | Function in Research | Application Example |
|---|---|---|
| 2-Mercaptoethanol | Radical initiator/scavenger | Biomimetic models of radical reactions [15] |
| Cholesteryl esters | Lipid substrate model | Studying radical-induced isomerization [15] |
| Lipoxygenase | Enzyme catalyst | Studying enzymatic oxidation pathways [9] |
| Internal standards (2-chlorophenylalanine, D₄-glutamic acid) | Metabolomic quantification | LC-MS normalization and quantification [9] |
| Acyl peroxides | Chemical initiator | Thermal generation of radical species [12] |
| Silver-TLC plates | Separation of geometric isomers | Analysis of trans lipid isomers [15] |
| Free fatty acids | Rancidity indicators | Acid value determination [9] |
Visualization of Autoxidation Mechanisms and Workflows
Free Radical Chain Reaction Mechanism in Autoxidation
Experimental Workflow for Rancidity Characterization
The free radical chain reaction mechanism of autoxidation, with its distinct initiation, propagation, and termination phases, provides the fundamental chemical framework for understanding lipid oxidation and food rancidity development. This mechanistic understanding enables researchers to identify critical control points for intervention strategies aimed at preserving food quality and extending shelf-life. The experimental protocols and analytical approaches outlined in this work provide researchers with robust methodologies for investigating autoxidation processes in diverse food systems, from model biomimetic environments to complex food matrices like pearl millet flour.
The implications of this research extend beyond fundamental chemical mechanisms to practical applications in food science, nutrition, and public health. By identifying specific metabolic biomarkers associated with rancidity progression, such as the 25 metabolites identified in pearl millet (including phytol, ethanolamine, chlorophyllide b, and glucoside derivatives), researchers can develop rapid assessment tools and targeted preservation technologies [9]. This integrated approach—connecting fundamental chemical mechanisms with practical food stability challenges—represents the future of lipid oxidation research, with significant potential for reducing food waste, enhancing nutritional quality, and improving global food security.
Lipid oxidation significantly impacts food quality, nutritional value, and safety. This technical guide delineates the distinct mechanistic pathways of auto-oxidation and photo-oxidation, emphasizing how each process generates characteristic hydroperoxide isomer profiles. Through detailed experimental protocols and analytical methodologies, we provide researchers with tools to differentiate these oxidation pathways. The findings are contextualized within food rancidity research, offering critical insights for developing effective stabilization strategies to preserve lipid-containing products.
Lipid oxidation is a paramount cause of food spoilage, leading to rancidity, degradation of nutritional quality, and generation of potentially harmful compounds [4]. Unsaturated fatty acids in fats and oils are particularly susceptible to oxidative deterioration. This review focuses on two primary oxidation pathways: auto-oxidation, a spontaneous free-radical chain reaction, and photo-oxidation, a light-induced process that can proceed via distinct mechanisms [16] [17]. Understanding the nuanced differences between these pathways is crucial for food scientists and product developers. The type of oxidation dictates the specific hydroperoxide isomers formed during the initial stages, which subsequently decompose into various volatile and non-volatile secondary products responsible for off-flavors, odors, and diminished shelf life [4] [18]. By identifying the dominant oxidation pathway through its isomer signature, targeted antioxidant strategies can be implemented to mitigate rancidity and maintain product integrity.
Fundamental Mechanisms
The initiation and propagation of lipid oxidation follow fundamentally different pathways depending on the presence of light and photosensitizers.
Auto-Oxidation: A Radical Chain Reaction
Auto-oxidation is a self-sustaining, free-radical chain reaction primarily initiated by heat, trace metals, or other pro-oxidants, rather than light [4] [18]. It proceeds through three classical stages:
- Initiation: The reaction begins with the abstraction of a hydrogen atom from a bis-allylic methylene group (e.g., in linoleic acid) of an unsaturated fatty acid (RH), forming a carbon-centered lipid radical (R•). This step requires an initial radical source.
- Propagation: The lipid radical (R•) rapidly reacts with molecular oxygen (³O₂) to form a lipid peroxyl radical (ROO•). This highly reactive species can then abstract a hydrogen from an adjacent lipid molecule (RH), generating a lipid hydroperoxide (ROOH) and a new carbon-centered radical (R•), thereby propagating the chain reaction.
- Termination: The reaction chain concludes when two radicals combine to form non-radical products [4].
A key characteristic of auto-oxidation is that the radical intermediates are resonance-stabilized. For instance, in linoleic acid, abstraction of a bis-allylic hydrogen leads to a pentadienyl radical that delocalizes, resulting in the formation of specific conjugated diene hydroperoxide isomers [4].
Photo-Oxidation: Pathways Involving Light
Photo-oxidation requires light absorption by a photosensitizer (e.g., chlorophyll, riboflavin, or synthetic dyes), which becomes excited and then engages in one of two primary pathways [16] [19] [17]:
- Type I (Contact-Dependent Pathway): The excited triplet-state photosensitizer (³Sen) reacts directly with a substrate (e.g., the lipid) via electron or hydrogen atom transfer, generating lipid radicals. These radicals then initiate a free-radical chain reaction analogous to auto-oxidation.
- Type II (Contact-Independent Pathway): The excited triplet-state photosensitizer (³Sen) transfers its energy directly to ground-state molecular oxygen (³O₂), generating singlet oxygen (¹O₂) [16] [19]. Singlet oxygen is an electrophilic excited state that reacts rapidly with the double bonds of unsaturated lipids via a concerted "ene" reaction.
This "ene" reaction is non-radical in nature and does not involve hydrogen abstraction. It leads to a shift of the double bond and the formation of hydroperoxides with the hydroperoxyl group on one of the allylic carbons. Crucially, because the reaction proceeds through a different mechanism, photo-oxidation can produce a wider array of hydroperoxide isomers compared to auto-oxidation, including isomers that are not formed during the radical-mediated auto-oxidation process [20].
The following diagram illustrates the core mechanistic differences between these pathways:
Characteristic Hydroperoxide Isomers
The distinct mechanisms of auto-oxidation and photo-oxidation directly dictate the isomeric profile of the resulting hydroperoxides, providing a chemical fingerprint to identify the dominant degradation pathway.
Isomer Profiles from Different Pathways
Docosahexaenoic acid (DHA) serves as an excellent model to illustrate these differences, as it is highly unsaturated and prone to oxidation. The table below summarizes the characteristic DHA hydroperoxide (DHA;OOH) isomers formed via each pathway.
Table 1: Characteristic DHA Hydroperoxide Isomers Formed via Auto-Oxidation and Photo-Oxidation [20]
| Oxidation Mechanism | Reactive Species | Characteristic DHA;OOH Isomers |
|---|---|---|
| Auto-Oxidation | Radicals (ROO•) | Formed via abstraction of bis-allylic hydrogen: 4-, 7-, 8-, 10-, 11-, 13-, 14-, 16-, 17-, 20-OOH |
| Photo-Oxidation (Type II) | Singlet Oxygen (¹O₂) | Includes all auto-oxidation isomers PLUS isomers unique to the 'ene' reaction with double bonds: 5-OOH and 19-OOH |
The presence of 5-OOH and 19-OOH isomers is a definitive marker for singlet oxygen-mediated photo-oxidation, as these specific isomers are not generated through the radical-based auto-oxidation pathway [20]. Similar principles apply to other unsaturated fatty acids; for example, singlet oxygen oxidation of oleic acid yields a mixture of 9-, 10-, and other positional isomers, some of which are non-conjugated, unlike the specific conjugated isomers formed from auto-oxidation.
Comparative Analysis of Isomer Formation
The following diagram visualizes the formation of these diagnostic isomers in DHA, highlighting the carbon atoms attacked by each mechanism:
Experimental Protocols for Isomer Analysis
Direct analysis of hydroperoxide isomers is essential for identifying oxidation pathways. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) provides the requisite sensitivity and selectivity.
Sample Preparation and Oxidation Induction
This protocol outlines the procedure for analyzing esterified lipids in complex matrices like mackerel [20].
- Lipid Extraction: Homogenize the food sample (e.g., 300 mg mackerel). Extract total lipids using a modified Folch method (chloroform:methanol, 2:1 v/v). Separate neutral lipids (triacylglycerols, TGs) and phospholipids (PLs) using solid-phase extraction on an aminopropyl cartridge. Elute neutral lipids with chloroform/2-propanol (2:1, v/v) and phospholipids with methanol. Dry fractions under a stream of N₂ gas and reconstitute in appropriate solvents for storage at -80 °C under N₂ [20].
- Induction of Photo-oxidation: Dissolve the purified lipid of interest (e.g., PC 16:0/22:6) in methanol. Add a photosensitizer like Rose Bengal (final concentration ~7.5 µg/mL). Place the solution on ice to mitigate thermal side reactions. Irradiate with a white LED light source (e.g., 6000 lux) for a defined period (e.g., 2 hours) to generate photo-oxidation products [20].
- Induction of Auto-oxidation (Thermal Oxidation): Incubate the purified lipid in the dark at elevated temperatures (e.g., 37-60 °C) for a defined period. This thermal energy promotes the radical chain reaction of auto-oxidation without light-induced pathways [21].
LC-MS/MS Analysis and Isomer Discrimination
The following workflow and table detail the critical analytical steps and reagents.
- Chromatography: Use a reversed-phase C18 column. For phospholipids like PC-DHA;OOH, employ isocratic elution with methanol/water (95:5, v/v). For neutral lipids like TG-DHA;OOH, use a binary gradient with methanol and 2-propanol [20].
- Mass Spectrometry Detection: Operate the mass spectrometer in positive ion mode. Detect lipids as sodium adducts ([M+Na]⁺) by post-column infusion of a sodium acetate solution. For isomer identification, use Multiple Reaction Monitoring (MRM) based on characteristic fragmentation patterns. Monitor specific α-cleavage fragments relative to the hydroperoxide group, which provides a unique fingerprint for each isomer's position, enabling direct discrimination [20].
Table 2: Research Reagent Solutions for Hydroperoxide Isomer Analysis [20]
| Reagent / Instrument | Function / Application |
|---|---|
| Rose Bengal | Photosensitizer dye used to induce Type II photo-oxidation via singlet oxygen generation. |
| Chloroform:MeOH (2:1 v/v) | Solvent system for the modified Folch method, used for total lipid extraction from food matrices. |
| Aminopropyl Cartridge | Solid-phase extraction column for fractionating total lipids into neutral lipids and phospholipids. |
| Sodium Acetate Solution | Post-column infusion reagent to promote the formation of sodium adducts ([M+Na]⁺) for sensitive MS detection. |
| C18 LC Column | Reversed-phase chromatography column for separating lipid hydroperoxide isomers prior to MS analysis. |
| Tandem Mass Spectrometer | Core instrument for identifying and quantifying isomeric hydroperoxides via MRM and characteristic α-cleavage fragments. |
The experimental workflow from sample to result is summarized below:
Implications for Food Rancidity Research
The differentiation of oxidation pathways has profound implications for assessing food quality and developing preservation strategies.
Different hydroperoxide isomers have varying stabilities and decomposition pathways, leading to different profiles of secondary oxidation products (aldehydes, ketones) that define the sensory attributes of rancidity [4]. Identifying the dominant pathway allows for the rational selection of antioxidants. For example, if photo-oxidation is the primary issue, singlet oxygen quenchers (e.g., carotenoids) or light-blocking packaging are optimal. If auto-oxidation dominates, radical scavengers (e.g., tocopherols, BHT, BHA) are more effective [5] [18]. Research on milk triacylglycerols has demonstrated that light exposure generates a distinct hydroperoxide isomer profile compared to thermal treatment, directly impacting flavor stability [21]. Similarly, in DHA-rich mackerel, radical oxidation was found to progress even under refrigeration, highlighting the need for effective stabilization beyond just light protection [20].
Auto-oxidation and photo-oxidation are distinct chemical processes that generate unique hydroperoxide isomer fingerprints. Auto-oxidation, a radical-mediated chain reaction, produces a specific set of isomers derived from bis-allylic hydrogen abstraction. In contrast, photo-oxidation, particularly the Type II singlet oxygen pathway, produces a broader isomeric profile, including isomers that serve as definitive biomarkers for light-induced damage. Modern LC-MS/MS methodologies enable researchers to differentiate these isomers directly in complex food matrices. This precise mechanistic understanding is fundamental for diagnosing the root cause of rancidity, enabling targeted antioxidant strategies, and ultimately preserving the sensory and nutritional quality of lipid-containing foods.
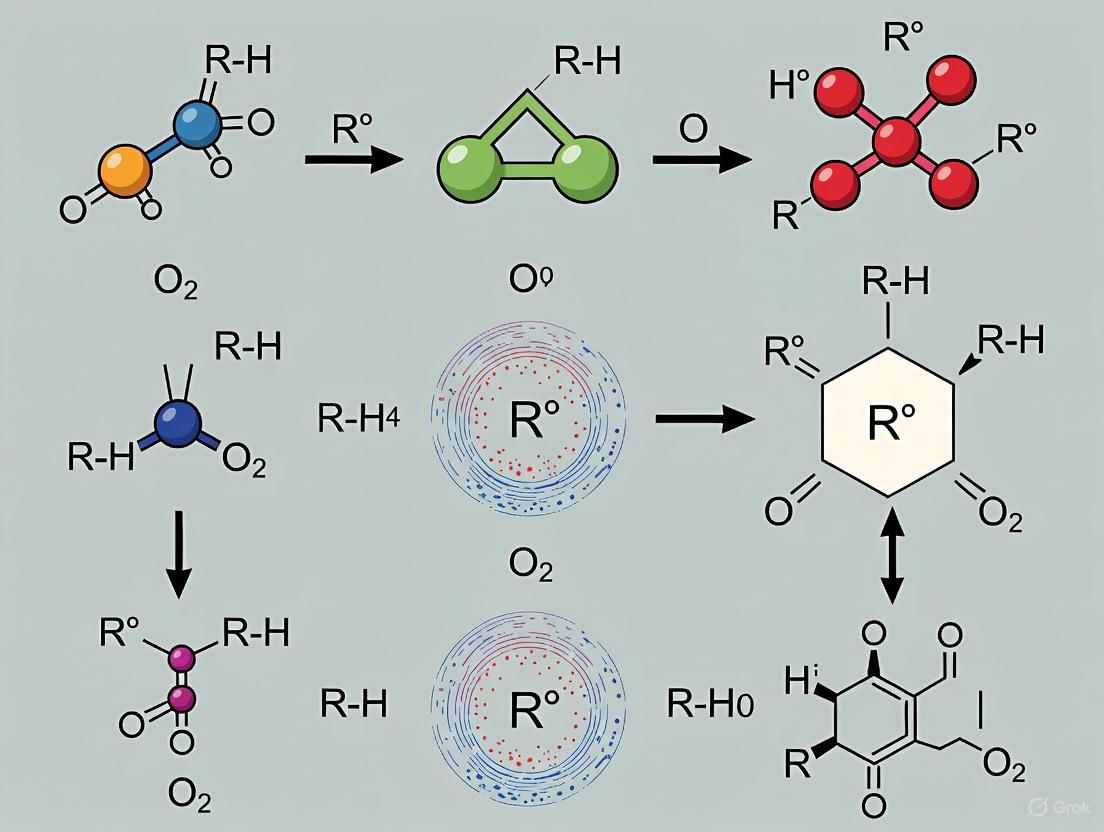
Lipid oxidation is a fundamental chemical process that represents the most significant chemical threat to food shelf life, quality, safety, and wholesomeness [22]. This complex series of reactions begins when unsaturated lipids in foods become degraded through exposure to oxygen, light, and heat, leading to the formation of undesirable flavors, aromas, and potentially toxic compounds [5] [23]. The process not only causes depreciation of organoleptic quality and shelf life but also directly contributes to increased food waste and economic losses [23]. For researchers and drug development professionals, understanding the precise pathways and products of lipid oxidation is crucial for developing effective stabilization strategies and assessing potential health implications.
The oxidation of lipids progresses via free-radical propagated chain reactions that are typically autocatalytic in nature [4] [24]. These reactions are initiated when unsaturated fatty acids react with molecular oxygen in the presence of initiators such as light, heat, or metal ions [23]. The susceptibility of fatty acids to oxidation increases with their degree of unsaturation due to progressively lower bond dissociation energies of methylene-interrupted carbons [23]. This makes polyunsaturated fatty acids (PUFAs), increasingly promoted in contemporary foods for health reasons, particularly vulnerable to oxidative degradation [22].
Within the context of food rancidity research, the transformation of hydroperoxides to aldehydes and ketones represents a critical pathway that directly impacts food quality and safety. This technical guide examines the key oxidation products formed during lipid degradation, with particular focus on their formation pathways, analytical detection methods, and significance in evaluating food stability and quality.
Mechanisms of Lipid Oxidation and Key Product Formation
The Three-Phase Oxidation Process
Lipid oxidation occurs through a well-characterized three-step process comprising initiation, propagation, and termination phases [5]. Each phase generates distinct products that serve as markers for assessing the extent and progression of oxidation:
Initiation Phase: The formation of free radicals begins and accelerates through hydrogen abstraction from lipid molecules, particularly at bis-allylic positions in polyunsaturated fatty acids [5]. This initial phase produces lipid radicals (L•) that react rapidly with molecular oxygen.
Propagation Phase: A chain reaction of high-energy molecules, including variations of free radicals and oxygen, propagates exponentially if not controlled [5]. During this phase, the rate of peroxide radical formation reaches equilibrium with the rate of decomposition, forming a characteristic bell-shaped curve when measured over time.
Termination Phase: The starting material becomes consumed, and peroxide radicals decompose into secondary oxidation by-products including esters, short-chain fatty acids, polymers, alcohols, ketones, and aldehydes [5]. It is these secondary oxidation by-products, particularly the aldehydes and ketones, that negatively affect food quality and sensory properties.
Formation Pathways of Primary Oxidation Products
Hydroperoxides (LOOHs) represent the main primary products of lipid auto-oxidation [4]. Their formation mechanism varies depending on the specific fatty acid substrate:
Oleic acid oxidation generates two allylic radicals through hydrogen abstraction at C8 and C11 positions, leading to the formation of 8-, 9-, 10-, and 11-allyl hydroperoxides [4] [3].
Linoleic acid auto-oxidation involves doubly reactive penta-dienyl radicals formed by abstraction from the allylic groups of C11, producing conjugated 9- and 13-diene hydroperoxides [4] [3].
Linolenic acid forms two penta-dienyl radicals by abstracting hydrogen on the C11 and C14 methylene groups [4] [3].
The degradation pathways of these hydroperoxides are highly dependent on temperature, pressure, and oxygen concentration [4]. Hydroperoxide cleavage generates alkoxy and hydroxyl radicals through homogeneous cleavage of the O-O bond, with the alkoxy radicals subsequently undergoing cleavage on the C-C bond to form aldehydes and vinyl radicals or unsaturated aldehydes and alkyl radicals [4] [3]. Among the volatile organic compounds generated through these pathways, aldehydes represent the essential critical aroma substances responsible for the characteristic odors associated with rancid fats and oils [4].
Figure 1: Lipid Oxidation Pathway from Initiation to Secondary Products. This diagram illustrates the free radical chain reaction mechanism of lipid oxidation, showing the progression from unsaturated lipids through hydroperoxide formation to aldehyde and ketone generation.
Critical Transition: From Hydroperoxides to Carbonyl Compounds
The transition from hydroperoxides to aldehydes and ketones represents a critical juncture in lipid oxidation that significantly impacts food quality. Recent research has identified the existence of a critical hydroperoxide concentration (CCLOOH) between 38-50 mmol/kg in food emulsions such as mayonnaise, at which point a rapid acceleration of aldehyde generation occurs [24]. This concentration threshold triggers enhanced secondary oxidation mechanisms, making the rapid acceleration of aldehydes imminent.
The specific aldehydes formed depend on the parent fatty acid and degradation pathway:
- Malondialdehyde (MDA) originates from fatty acids containing three or more double bonds [4]
- Hexanal derives predominantly from n-6 fatty acids [24]
- 4-Hydroxy-2-hexenal and 4-Hydroxy-2-nonenal form through more complex degradation pathways [25]
These carbonyl compounds are chemically reactive and can participate in further reactions with proteins and other food components, leading to protein aggregation, changes in food texture, loss of nutritional value, and formation of potentially harmful compounds [4] [23].
Analytical Methods for Detection and Quantification
Assessment of Primary Oxidation Products
The analytical techniques for detecting lipid oxidation products correspond to measuring specific oxidation products and their consequences [4]. For primary oxidation products, the following methods are commonly employed:
Peroxide Value (PV) is the most widely used method for determining peroxide content in foods, particularly meat products [4]. The iodometric and ferric thiocyanate methods directly measure the degree of hydroperoxides formed by oxidation [4]. While the iodometric assay is highly sensitive and accurate, it requires stringent precautions to minimize oxygen in the reaction solution and prevent substances that may induce hydroperoxide decomposition or react with iodine [4].
Conjugated Diene Analysis measured at 233 nm is suitable for polyunsaturated fatty acid-containing foods [4]. This method provides actual values of low-density lipoprotein oxidation during the early stages and offers convenience and low cost, though it depends on lipoprotein composition and size and may struggle to detect small conjugated dienes [4].
High-Performance Liquid Chromatography (HPLC) methods, particularly when coupled with chemiluminescence detection (CL-HPLC), enable sensitive determination of lipid hydroperoxides with specificity to hydroperoxide groups [26]. This system can detect as low as 7 nmol of phosphatidylcholine hydroperoxide and has revealed the presence of 28-431 pmol/ml of PCOOH in healthy human plasma [26].
Nuclear Magnetic Resonance (NMR) spectroscopy has emerged as a powerful technique for simultaneously quantifying both primary and secondary lipid oxidation products [24]. ¹H NMR methods provide rapid and accurate quantification of the bulk of hydroperoxides and non-volatile aldehydes, enabling comprehensive tracking of oxidation progression [24].
Assessment of Secondary Oxidation Products
Secondary oxidation products, particularly aldehydes and ketones, are typically measured using different approaches:
p-Anisidine Value (p-AV) determines the amount of reactive aldehydes and ketones in the lipid portion of a sample [5]. The compound p-anisidine reacts readily with aldehydes and ketones, and the reaction product is measured using a colorimeter [5]. This method is particularly useful for detecting non-volatile carbonyl compounds that remain in the oil after heating.
Thiobarbituric Acid Reactive Substances (TBARS) assay measures aldehydes (primarily malondialdehyde) created during lipid oxidation [4] [5]. This analysis is primarily useful for low-fat samples, as the whole sample can be analyzed rather than just the extracted lipids [5]. The production of MDA-TBA adducts is detected at 532 nm [4].
Gas Chromatography (GC) methods effectively detect volatile compounds that indicate rancidity, including hexanal and other low-molecular-weight aldehydes and ketones [5] [24]. These methods offer high sensitivity and specificity for profiling the volatile compounds responsible for off-flavors and odors.
Electrochemical Methods represent emerging approaches for rapid assessment of oil quality. Recent studies have demonstrated that cyclic voltammetry, electrochemical impedance spectroscopy, and differential pulse voltammetry parameters show strong correlations with traditional chemical indicators, such as the DPV peak current at +0.2 V with p-anisidine value (r = 0.94, p < 0.001) [27].
Table 1: Analytical Methods for Key Oxidation Products
| Target Compound | Analytical Method | Principle | Detection Range/Limit | Applications |
|---|---|---|---|---|
| Hydroperoxides | Peroxide Value (PV) | Titration of peroxides | Varies by method; iodometric highly sensitive | Meat, edible oils, oil-based products |
| Hydroperoxides | Conjugated Diene Analysis | UV absorption at 233-234 nm | Convenient for early stage oxidation | PUFA-containing foods |
| Hydroperoxides | CL-HPLC | Chemiluminescence detection post-separation | 7 nmol for PCOOH | Biological samples, quantitative mechanism studies |
| Aldehydes | p-Anisidine Value (p-AV) | Colorimetric reaction with aldehydes | Suitable for objectionable flavors at low levels | Oils, fats, dark samples not ideal |
| Malondialdehyde | TBARS | Colorimetric reaction with TBA | 532 nm detection | Low-fat samples, meat products, fish |
| Volatile Aldehydes/Ketones | Gas Chromatography (GC) | Separation and detection of volatiles | High sensitivity and specificity | Profile volatile off-flavors, hexanal detection |
Advanced and Integrated Approaches
Contemporary research employs integrated analytical approaches to gain comprehensive understanding of lipid oxidation processes:
Total Oxidation (TOTOX) Value combines both primary and secondary oxidation measurements using the formula: TOTOX = 2 × PV + AV [25]. This integrated value provides a more complete picture of the overall oxidation state.
Machine Learning Applications have recently been applied to predict oxidation parameters. Random Forest models trained on electrochemical data have accurately predicted TOTOX values, achieving R² of 0.96 and RMSE of 2.18 for test sets [27]. Feature importance analysis revealed charge transfer resistance and DPV peak currents as the most influential predictors [27].
Accelerated Shelf-Life Testing (ASLT) is widely used in the food industry to assess oxidative stability of different formulations [24]. By storing products under elevated temperatures (e.g., 50°C), oxidation reactions are stimulated to enable faster assessment of stability and shelf-life [24].
Figure 2: Analytical Workflow for Lipid Oxidation Assessment. This diagram outlines the comprehensive approach to analyzing lipid oxidation products, from sample preparation through primary and secondary product analysis to advanced integrated methods and data interpretation.
Experimental Protocols for Key Analyses
Hydroperoxide Quantification by CL-HPLC
The CL-HPLC system enables sensitive determination of lipid hydroperoxides with specificity to hydroperoxide groups, using a chemiluminescence reaction selective for lipid hydroperoxides as the detection part of HPLC [26].
Reagents and Materials:
- Luminol-cytochrome c solution as hydroperoxide-specific luminescent reagent
- High-purity lipid hydroperoxide standards (e.g., phosphatidylcholine hydroperoxide)
- Normal phase HPLC system
- Single photon counting detector
Procedure:
- Extract lipids from samples using appropriate solvents (chloroform:methanol)
- Separate lipid classes by normal phase HPLC
- Post-column mixing with luminol-cytochrome c reagent
- Detect chemiluminescence using single photon counter
- Quantify based on standard curves of authentic hydroperoxides
Critical Notes:
- Detection limit: approximately 7 nmol of phosphatidylcholine hydroperoxide (PCOOH)
- Enables detection of 28-431 pmol/ml of PCOOH in healthy human plasma
- The chemiluminescence reaction is based on the oxidation of luminol by reaction with lipid hydroperoxides and cytochrome c, generating singlet oxygen that oxidizes luminol to produce light emission at 430 nm [26]
Accelerated Shelf-Life Testing with Predictive Modeling
This protocol enables prediction of aldehyde onset based on early hydroperoxide formation kinetics, significantly reducing assessment time from weeks to days [24].
Reagents and Materials:
- Food emulsion samples (e.g., mayonnaise)
- CDCl₃, DMSO-d6 with 4Å molsieves for NMR
- 600 MHz NMR spectrometer
- Temperature-controlled storage chambers
Procedure:
- Prepare emulsion samples with varying antioxidant formulations
- Store aliquots in sealed containers at accelerated conditions (50°C)
- Collect samples at regular intervals (e.g., daily for first week, then weekly)
- Separate oil phase by freeze-thaw and centrifugation (5 min at 17,000 × g)
- Dissolve oil phase (150 μL) in 450 μL 5:1 CDCl₃:DMSO-d6 solvent
- Acquire ¹H NMR spectra using single pulse and band selective experiments
- Quantify hydroperoxides and aldehydes based on characteristic signals
- Fit hydroperoxide concentration vs. time data to Foubert model: [ C(t) = \frac{C{\text{max}}}{1 + e^{-k(t - t{\text{mid}})}} ] where (C{\text{max}}) is maximum LOOH, (k) is rate constant, and (t{\text{mid}}) is midpoint time
- Determine critical LOOH concentration (CCLOOH, typically 38-50 mmol/kg)
- Predict aldehyde onset time as time when LOOH concentration reaches CCLOOH
Critical Notes:
- Foubert function better describes LOOH curvature than Gompertz function
- Model parameters can recognize antioxidant mechanisms at play
- Enables accurate prediction of aldehyde onset within several days rather than weeks
Table 2: Research Reagent Solutions for Lipid Oxidation Analysis
| Reagent/Instrument | Function | Application Examples | Technical Notes |
|---|---|---|---|
| Luminol-Cytochrome c | Chemiluminescence detection of hydroperoxides | CL-HPLC for lipid hydroperoxide quantification | Specific for hydroperoxide groups; enables sensitive detection |
| p-Anisidine Reagent | Colorimetric detection of aldehydes | p-Anisidine value for secondary oxidation | Reacts with α,β-unsaturated aldehydes; not suitable for dark samples |
| Thiobarbituric Acid (TBA) | Colorimetric detection of malondialdehyde | TBARS assay for lipid peroxidation extent | Measures primarily MDA; useful for low-fat samples |
| Deuterated Solvents (CDCl₃, DMSO-d6) | NMR analysis without hydrogen interference | ¹H NMR quantification of hydroperoxides and aldehydes | Enables simultaneous detection of multiple oxidation products |
| Fatty Acid Standards | Calibration and identification | GC analysis of fatty acid composition | Essential for quantitative analysis; purity critical |
| Antioxidants (Tocopherols, EDTA, Gallic Acid) | Oxidation inhibition studies | Mechanism studies and antioxidant efficacy | Concentrations typically 10-1000 ppm depending on antioxidant |
Implications for Food Quality and Research Applications
Impact on Food Systems
The progression from hydroperoxides to aldehydes and ketones has profound implications for food quality:
Sensory Deterioration: Aldehydes and ketones generated during lipid oxidation are responsible for the characteristic rancid odors and flavors that render foods unacceptable to consumers [5] [23]. Hexanal, in particular, is linked to the perceived off-taste and off-flavor of oxidized products [24].
Nutritional Quality: Lipid oxidation destroys critical nutrients, including essential fatty acids and fat-soluble vitamins [22]. The resulting products may also reduce protein digestibility and bioavailability through formation of protein aggregates [4].
Physical Properties: In food emulsions such as mayonnaise, lipid oxidation products can affect stability and texture [24]. Protein oxidation induced by lipid oxidation products can lead to protein aggregation, significantly affecting protein physicochemical characteristics and biological functions [4].
Health and Safety Considerations
Beyond quality implications, lipid oxidation products raise important health and safety concerns:
Toxic Products: Oxidation products accumulating to a certain extent could be detrimental to consumer health [4]. Some oxidation products, including certain epoxides and aldehydes, are known to be toxic and potentially mutagenic [22].
Cellular Effects: Studies have shown that oral ingestion of oxidized oil causes severe damage to immune tissues in animal models, with necrosis observed in lymphocytes located in the thymus, and significant decreases in thymus weight, spleen weight, and blood leucocytes number [26].
Chronic Disease Links: There is ample evidence showing that free radical oxidation plays an important role in many pathological situations, including many types of cancer, atherosclerosis, coronary heart disease, rheumatoid arthritis, and other degenerative diseases [23].
Research Significance and Future Directions
Understanding the pathways from hydroperoxides to aldehydes and ketones provides critical insights for:
Food Stabilization Strategies: Traditional practices of adding synthetic antioxidants are insufficient for contemporary foods reformulated with high polyunsaturated fatty acids for health, largely because oxidation in complex food systems involves more molecules than just lipids [22]. Understanding these pathways enables development of more effective stabilization approaches.
Analytical Method Development: Nearly all analyses in industry or research concentrate on how fast a food oxidizes while ignoring how it oxidizes and what types of damage results [22]. Current analyses miss key lipid oxidation products and do not account for broadcasting oxidation to other molecules, particularly proteins [22].
Predictive Modeling: The identification of critical hydroperoxide concentrations that trigger rapid aldehyde formation enables development of predictive models that can significantly reduce the time required for shelf-life assessment [24]. Machine learning approaches applied to electrochemical data show particular promise for rapid quality assessment [27].
The comprehensive understanding of key oxidation products from hydroperoxides to aldehydes and ketones remains essential for advancing food stability, quality, and safety while potentially providing insights relevant to oxidative processes in biological systems and disease pathogenesis.
Lipid oxidation represents a paramount challenge in food science, serving as the primary driver of quality deterioration in lipid-containing food matrices. This complex chemical process not only compromises the lipids themselves but also initiates a cascade of destructive events that negatively impact proteins, sensory attributes, and nutritional value. Within food systems, unsaturated fatty acids react with oxygen through various pathways to form highly reactive free radicals and lipid hydroperoxides, which subsequently decompose into a wide range of secondary products [4] [28]. These reactive intermediates propagate oxidative damage throughout the food matrix, leading to the development of off-flavors, degradation of color and texture, loss of essential nutrients, and formation of potentially toxic compounds [28].
The significance of lipid oxidation extends beyond mere food spoilage, as the process is intimately involved in co-oxidation reactions with proteins, fundamentally altering their structure and functionality [4]. Research interest in this field has grown substantially, with publications on lipid and protein oxidation increasing markedly over the past two decades, reflecting heightened recognition of its implications for food quality and human health [3]. Understanding these complex reaction mechanisms provides the foundational knowledge necessary for developing effective strategies to mitigate oxidative damage in food products, thereby extending shelf life, maintaining sensory appeal, and preserving nutritional integrity within the broader context of food rancidity research.
Fundamental Mechanisms of Lipid Oxidation
Lipid oxidation progresses through a well-defined series of chemical reactions that collectively contribute to the degradation of food matrices. The process typically occurs via three interconnected pathways—auto-oxidation, photo-oxidation, and enzymatic oxidation—with auto-oxidation representing the predominant free-radical chain reaction in most food systems [4].
The Free Radical Chain Reaction
Auto-oxidation proceeds through three distinct phases that collectively drive the oxidative deterioration of food matrices:
- Initiation: This initial phase involves the formation of free lipid radicals (L•) through the abstraction of a hydrogen atom from unsaturated fatty acid molecules (LH), typically catalyzed by factors such as heat, light, or metal ions [4]. The reaction begins as follows: LH → L• + H•.
- Propagation: During this phase, lipid radicals (L•) rapidly react with molecular oxygen (O₂) to form lipid peroxyl radicals (LOO•). These highly reactive intermediates then abstract hydrogen atoms from adjacent unsaturated fatty acids, generating lipid hydroperoxides (LOOH) and new lipid radicals (L•), thereby propagating the chain reaction [4]. The propagation reactions are represented as: L• + O₂ → LOO• and LOO• + LH → LOOH + L•.
- Termination: The chain reaction concludes when free radicals combine with one another to form non-radical products, effectively terminating the oxidative process [4]. Termination reactions include: LOO• + LOO• → LOOL + O₂, L• + L• → LL, and LOO• + L• → LOOL.
Table 1: Primary Lipid Oxidation Products Formed During Different Fatty Acid Oxidation
| Fatty Acid | Type | Primary Oxidation Products | Characteristic Features |
|---|---|---|---|
| Oleic Acid | Monounsaturated | 8-, 9-, 10-, and 11-allyl hydroperoxides | Formation of two allylic radicals with electrons delocalized at three carbon atoms [4] |
| Linoleic Acid | Polyunsaturated | Conjugated 9- and 13-diene hydroperoxides | Formation of penta-dienyl radicals with electrons delocalized at five carbon atoms [4] |
| Linolenic Acid | Polyunsaturated | Multiple hydroperoxide isomers | Formation of two penta-dienyl radicals by abstracting hydrogen on C11 and C14 methylene groups [4] |
Formation of Secondary Oxidation Products
Lipid hydroperoxides (LOOH), the primary products of lipid oxidation, are inherently unstable and decompose readily under the influence of heat, light, or metal catalysts. This decomposition generates alkoxy (LO•) and hydroxyl (HO•) radicals through homolytic cleavage of the peroxide bond [4]. These radicals subsequently undergo carbon-carbon bond cleavage, yielding volatile compounds including aldehydes, alkenes, alcohols, and ketones [4]. Among these secondary oxidation products, aldehydes such as malondialdehyde, 4-hydroxy-trans-2-nonenal, and hexenal are particularly significant due to their low odor thresholds and profound impact on sensory properties [4]. These carbonyl compounds are responsible for the characteristic rancid aromas and flavors associated with oxidized foods, and they also participate in further chemical interactions with proteins, pigments, and other food components, leading to co-oxidation phenomena [4].
Impact on Sensory Quality
The sensory degradation of food matrices resulting from lipid oxidation manifests through distinct changes in flavor, aroma, color, and texture. These alterations significantly reduce consumer acceptance and marketability of affected products.
Flavor and Aroma Deterioration
The development of undesirable flavors and aromas constitutes the most readily perceptible indicator of lipid oxidation. As polyunsaturated fatty acids undergo oxidation, they generate volatile compounds that impart characteristic off-flavors even at minute concentrations [28]. The decomposition of lipid hydroperoxides yields aldehydes, ketones, alcohols, and hydrocarbons that collectively create the sensory experience described as rancidity [4]. Specific volatile compounds produce distinct off-notes: omega-3 fatty acid oxidation generates fishy aromas, while omega-6 fatty acid oxidation produces grassy or painty odors [28]. In meat products, aldehydes derived from lipid oxidation contribute significantly to flavor deterioration and are closely associated with the development of warmed-over flavor [4]. The interaction between lipid oxidation products and Maillard reaction pathways further complicates flavor development, particularly in thermally processed foods [29].
Color and Texture Modifications
Lipid oxidation indirectly affects food color through co-oxidation of pigments and direct interaction with protein structures. Free radicals generated during lipid oxidation readily attack conjugated double bonds in color pigments such as carotenoids and myoglobin, leading to bleaching or discoloration [28]. In muscle foods, lipid oxidation products facilitate the oxidation of oxymyoglobin to metmyoglobin, resulting in an undesirable brownish-gray discoloration [4] [3]. Secondary lipid oxidation products, particularly unsaturated aldehydes, react with primary amino groups in proteins, potentially forming brown Maillard-type pigments that further alter food appearance [28].
Textural changes in oxidized food matrices primarily result from protein co-oxidation induced by lipid-derived free radicals and secondary carbonyl compounds. These interactions promote protein aggregation through covalent cross-linking, including the formation of disulfide bonds and carbonyl-amine bridges [4]. In meat systems, oxidative damage to myofibrillar proteins, especially myosin, leads to diminished water-holding capacity, increased toughness, and reduced juiciness [4] [29]. The structural alterations in proteins subsequently affect functional properties such as emulsification capacity, foam stability, and gelation, further compromising product quality and performance in complex food matrices [4].
Table 2: Sensory Impact of Specific Lipid Oxidation Products
| Oxidation Product | Class | Sensory Impact | Food Systems Where Commonly Found |
|---|---|---|---|
| Malondialdehyde | Aldehyde | Rancid, pungent odor | Meat, fish, edible oils [4] |
| 4-Hydroxy-trans-2-Nonenal | Aldehyde | Strong, disagreeable odor | Vegetable oils, meat products [28] |
| Hexanal | Aldehyde | Green, grassy odor | Vegetable oils, nuts, grains [4] |
| Acrolein | Aldehyde | Acrid, burning aroma | Heated oils, fried foods [28] |
| Crotonaldehyde | Aldehyde | Pungent, suffocating odor | Fried chips, fish, meat [28] |
Nutritional Loss and Health Implications
Lipid oxidation profoundly impacts the nutritional quality of food matrices through multiple mechanisms that extend beyond mere sensory deterioration. The process diminishes nutritional value by degrading essential fatty acids, fat-soluble vitamins, and protein quality, while simultaneously generating potentially harmful compounds.
Degradation of Essential Nutrients
The oxidative process preferentially targets polyunsaturated fatty acids (PUFAs), particularly nutritionally essential omega-3 and omega-6 fatty acids, thereby reducing their bioavailability and biological efficacy [28]. The destruction of these essential lipids represents a significant nutritional loss, as they play crucial roles in human physiology, including inflammatory regulation, neurological function, and cardiovascular health. Concurrently, lipid oxidation accelerates the degradation of fat-soluble vitamins (A, D, E, and K) and carotenoids, which are highly susceptible to radical-mediated degradation [28]. The oxidation of proteins by lipid-derived reactive species diminishes their nutritional value by reducing the bioavailability of essential amino acids, particularly lysine, methionine, cysteine, and histidine [4]. This oxidative modification compromises protein digestibility and alters amino acid profiles, thereby diminishing the protein quality score of affected foods [4].
Formation of Potentially Toxic Compounds
The secondary oxidation products generated during lipid oxidation include various aldehydes that demonstrate potential toxicological effects in biological systems. Among these, α,β-unsaturated aldehydes such as acrolein, crotonaldehyde, 4-hydroxy-trans-2-nonenal (HNE), and 4-hydroxy-trans-2-hexenal (HHE) exhibit particularly concerning biological activities [28]. These reactive carbonyl species can form covalent adducts with cellular proteins, DNA, and phospholipids, potentially disrupting normal physiological functions [28]. Acrolein, produced through linoleic acid oxidation, has been associated with myocardial oxidative stress, cardiomyopathy, and vascular dysfunction in animal studies [28]. Crotonaldehyde, detected in various fried and processed foods, has demonstrated hepatocarcinogenic potential in rodent models through the formation of DNA adducts [28]. HNE and HHE, derived from omega-6 and omega-3 fatty acid oxidation respectively, have shown cytotoxic effects and the ability to induce thymic necrosis in experimental animals [28].
The human health implications of dietary lipid oxidation products remain an area of active investigation, as biological systems possess sophisticated antioxidant defenses and repair mechanisms. However, evidence suggests that consumed oxidation products can be absorbed through the gastrointestinal tract, with unsaturated aldehydes demonstrating greater bioavailability than lipid hydroperoxides [28]. Recent research indicates that lipid oxidation products may adversely affect gut health by altering microbiota composition and promoting colonic inflammation, potentially increasing susceptibility to conditions such as colorectal cancer [28].
Protein Co-oxidation: Mechanisms and Consequences
The interplay between lipid and protein oxidation represents a critical dimension of food matrix deterioration, with lipid-derived reactive species initiating and propagating protein oxidation through various mechanistic pathways.
Mechanisms of Protein Co-oxidation
Protein co-oxidation occurs when reactive intermediates generated during lipid oxidation attack and modify protein structures. Free radicals, including lipid peroxyl (LOO•) and alkoxyl (LO•) radicals, abstract hydrogen atoms from susceptible amino acid side chains, generating protein-centered radicals that subsequently undergo further reactions [4]. These protein radicals can cross-link with other protein molecules or with lipid radicals, forming complex aggregates that alter protein functionality. Simultaneously, secondary lipid oxidation products, particularly reactive aldehydes such as malondialdehyde (MDA), 4-hydroxy-2-nonenal (HNE), and hexenal, form covalent adducts with nucleophilic amino acid residues (e.g., lysine, cysteine, histidine) through Michael addition and Schiff base reactions [4]. These modifications lead to the formation of protein carbonyl groups, loss of sulfhydryl groups, and the generation of advanced lipoxidation end products (ALEs) that parallel advanced glycation end products in their complexity and stability [4].
The initial process of lipid oxidation generates free radicals that subsequently initiate protein oxidation through several interconnected pathways as illustrated below:
Structural and Functional Consequences
Protein oxidation induced by lipid-derived reactive species profoundly affects protein structure at primary, secondary, and tertiary levels, subsequently altering functional properties critical to food quality. Key structural modifications include:
- Carbonyl Formation: Introduction of carbonyl groups on amino acid side chains serves as a hallmark of protein oxidation and correlates with loss of protein functionality [4].
- Sulfhydryl Loss: Oxidation of cysteine residues results in disulfide bond formation, both intra- and intermolecularly, leading to protein aggregation [4].
- Amino Group Modification: Lysine residues react with lipid-derived aldehydes, diminishing protein nutritional quality and creating protein cross-links [4].
- Protein Aggregation: Covalent cross-linking through disulfide bonds, tyrosine dimerization, and aldehyde-mediated bridges generates high molecular weight aggregates with reduced solubility [4].
These structural alterations manifest in significant functional changes that impact food quality. Oxidized proteins typically demonstrate reduced digestibility due to enzyme-resistant cross-links and aggregation, diminishing their nutritional value [4]. Technological functionalities such as emulsification capacity, foam stability, water-holding capacity, and gelation properties are often impaired, compromising performance in processed food applications [4]. In muscle foods, protein oxidation contributes to texture deterioration, including increased toughness and reduced juiciness, while in cereal systems it affects dough handling properties and final product texture [4].
Analytical Methods and Assessment Protocols
Comprehensive evaluation of lipid oxidation and its impact on food matrices requires a multifaceted analytical approach targeting various oxidation products and their effects on coexisting food components.
Assessment of Lipid Oxidation
The complex nature of lipid oxidation necessitates multiple analytical methods to capture the full scope of oxidative deterioration throughout the process. The following workflow outlines a comprehensive analytical strategy for monitoring lipid oxidation:
Table 3: Analytical Methods for Assessing Lipid Oxidation in Food Matrices
| Analysis Target | Method | Principle | Applications | Advantages/Limitations |
|---|---|---|---|---|
| Primary Products | Peroxide Value (PV) | Titration-based measurement of hydroperoxide content [4] | Oils, meat, fish, edible insects | Highly sensitive and accurate for early oxidation; requires oxygen-free conditions [4] |
| Primary Products | Conjugated Dienes | UV absorption at 233 nm [4] | Polyunsaturated fatty acid-containing foods | Convenient and low-cost; limited sensitivity for small dienes [4] |
| Secondary Products | Thiobarbituric Acid Reactive Substances (TBARS) | Measurement of malondialdehyde-thiobarbituric acid complex at 532 nm [4] | Meat, fish, edible insects | Well-established; may lack specificity due to interference [4] |
| Secondary Products | p-Anisidine Value | Colorimetric determination of aldehydes [5] | Oils and oil-based products | Simple calculation; problematic for dark samples and omega-3-rich oils [4] |
| Volatile Compounds | Gas Chromatography-Mass Spectrometry (GC-MS) | Separation and identification of volatile oxidation products [4] | All lipid-containing foods | Sensitive and specific; requires specialized equipment [4] |
| Oxidative Stability | Oxidative Stability Index (OSI) | Accelerated oxidation under heat and air flow [5] | Fats, oils, antioxidant efficacy testing | Predicts shelf-life; evaluates antioxidant effectiveness [5] |
Assessment of Protein Oxidation and Aggregation
Evaluating protein co-oxidation requires specific analytical approaches targeting the structural modifications induced by lipid-derived reactive species:
- Protein Carbonyl Content: Quantification of protein-bound carbonyl groups provides a well-established marker of protein oxidation, typically measured spectrophotometrically after derivatization with 2,4-dinitrophenylhydrazine (DNPH) [4].
- Sulfhydryl Group Analysis: Determination of free thiol groups using Ellman's reagent or similar methodologies monitors the oxidation of cysteine residues to disulfide bonds or higher oxidation states [4].
- Protein Aggregation Assessment: Size-exclusion chromatography, SDS-PAGE, and dynamic light scattering techniques evaluate the formation of high molecular weight protein aggregates resulting from oxidative cross-linking [4].
- Amino Acid Analysis: Chromatographic quantification of specific amino acids, particularly lysine, methionine, cysteine, and histidine, assesses oxidative losses and modifications [4].
- Digestibility Studies: In vitro simulated gastrointestinal digestion models evaluate the impact of oxidative modifications on protein digestibility and amino acid bioavailability [4].
Advanced Lipidomic Approaches
Recent advances in analytical technologies have enabled more comprehensive assessment of lipid oxidation through lipidomic approaches. Liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) provides detailed characterization of lipid molecular species and their oxidation products [30]. This methodology allows for the identification and quantification of hundreds to thousands of individual lipid compounds, offering unprecedented insight into oxidative pathways and patterns [30]. Lipidomics has revealed distinct profiles between raw and thermally processed fats, demonstrating significant alterations in phospholipids including phosphatidylcholines (PCs), phosphatidylethanolamines (PEs), and sphingomyelins (SMs) following thermal extraction and oxidative stress [30]. This advanced approach facilitates the identification of specific lipid oxidation markers and provides a more complete understanding of oxidative deterioration at the molecular level.
The Scientist's Toolkit: Essential Research Reagents and Materials
Investigating lipid oxidation and its impact on food matrices requires specialized reagents, standards, and analytical materials to ensure accurate and reproducible results.
Table 4: Essential Research Reagents and Materials for Lipid Oxidation Studies
| Reagent/Material | Function/Application | Specific Examples | Technical Considerations |
|---|---|---|---|
| Radical Initiators | Generate free radicals in model systems to study oxidation mechanisms | 2,2'-Azobis(2-amidinopropane) dihydrochloride (AAPH) [4] | Water-soluble; generates peroxyl radicals at predictable rates |
| Metal Catalysts | Initiate and accelerate lipid oxidation through redox cycling | FeCl₂, FeCl₃, CuSO₄ [30] | Concentration-dependent effects; require careful handling |
| Antioxidants | Reference compounds for oxidation inhibition studies | BHT, BHA, tocopherols, ascorbic acid [5] | Varying mechanisms (radical scavenging, metal chelation) |
| Lipid Standards | Calibration and quantification in analytical methods | Methyl linoleate, trilinolein, fatty acid standards [31] | Purity critical for accurate quantification |
| Derivatization Reagents | Chemical modification for detection and quantification | DNPH (carbonyls), Ellman's reagent (thiols), TBA (malondialdehyde) [4] | Reaction conditions must be carefully controlled |
| Oxidation Probes | Fluorescent or colorimetric detection of oxidation products | Thiobarbituric acid, p-anisidine [4] [5] | Potential interference from food matrix components |
| Chromatography Standards | Identification and quantification of specific oxidation products | 4-Hydroxynonenal, hexanal, hydroperoxide standards [4] | Authentic standards often unstable; require proper storage |
| Solid Phase Extraction | Sample clean-up and concentration prior to analysis | C18, silica, aminopropyl cartridges [30] | Improves sensitivity and reduces matrix effects |
Experimental Protocols for Key Methodologies
Standardized experimental protocols ensure reproducibility and comparability of results across different investigations of lipid oxidation and protein co-oxidation.
Hydroxyl Radical-Induced Oxidation System
This widely employed experimental approach generates hydroxyl radicals through Fenton-like reactions to induce controlled oxidation in biological and food samples:
- Reagent Preparation: Prepare phosphate buffer solution (0.02 mol/L, pH 6.0) containing 0.01 mmol/L FeCl₃ and 0.1 mmol/L ascorbic acid as described by Cao et al. (2020) [30].
- Oxidant Addition: Add H₂O₂ at varying concentrations (0, 10, 20, or 30 mmol/L) to establish a concentration-dependent oxidation response [30].
- Sample Treatment: Mix fat or protein samples with the oxidation buffer in a ratio of 1:4 (w/v) to ensure uniform exposure to the oxidative environment [30].
- Incubation: Subject samples to oxidation at 4°C for 24 hours to simulate extended storage conditions while minimizing microbial growth [30].
- Reaction Termination: Arrest oxidation by adding 1 mmol/L EDTA to chelate metal catalysts and prevent continued radical generation [30].
- Washing: Remove excess oxidants by washing samples twice with phosphate buffer (0.02 mol/L, pH 6.0) prior to subsequent analysis [30].
Peroxide Value Determination
The peroxide value (PV) quantifies primary lipid oxidation products through an iodometric titration approach:
- Lipid Extraction: Homogenize approximately 2 g of sample with 15 mL chloroform-methanol (2:1, v/v) containing 0.05% (w/v) BHT as antioxidant at 12,000 rpm for 30 seconds [30].
- Phase Separation: Add 3 mL of 0.5% (w/v) NaCl solution, vortex vigorously for 15 seconds, and centrifuge at 3,000 × g for 10 minutes at 4°C [30].
- Colormetric Reaction: Collect 5 mL of the bottom organic phase and mix with equal volume of chloroform-methanol (2:1, v/v), 25 μL of 30% (w/v) ammonium thiocyanate, and 25 μL of FeCl₂ solution [30].
- Incubation and Measurement: Incubate the reaction mixture for 20 minutes at 20 ± 2°C and measure absorbance at 500 nm against a blank containing all reagents except the sample [30].
- Calculation: Determine peroxide value using the formula: PV (meq/kg) = (k × (Aₛ - Aᵦ) × 1000) / (55.84 × m × 2), where k is the standard curve slope, Aₛ is sample absorbance, Aᵦ is blank absorbance, and m is sample mass in grams [30].
Lipidomic Analysis by LC-MS/MS
Comprehensive lipid profiling using liquid chromatography tandem mass spectrometry provides detailed molecular-level information on oxidative modifications:
- Lipid Extraction: Employ methyl-tert-butyl ether (MTBE) or chloroform-methanol based extraction methods with appropriate internal standards for lipid class quantification [30].
- Chromatographic Separation: Utilize reversed-phase C18 columns (e.g., 1.7 μm, 2.1 × 100 mm) with mobile phases consisting of (A) acetonitrile-water (60:40, v/v) and (B) isopropanol-acetonitrile (90:10, v/v), both containing 10 mmol/L ammonium formate [30].
- Mass Spectrometric Detection: Operate mass spectrometer in both positive and negative ionization modes with data-dependent acquisition (DDA) or data-independent acquisition (DIA) to capture comprehensive lipid information [30].
- Data Processing: Use specialized lipidomics software (e.g., LipidSearch, Skyline) for peak alignment, identification, and quantification based on accurate mass and retention time [30].
- Statistical Analysis: Apply multivariate statistical methods (PCA, OPLS-DA) to identify significantly altered lipid species between control and oxidized samples [30].
Lipid oxidation profoundly impacts food matrices through multifaceted mechanisms that compromise sensory quality, nutritional value, and protein functionality. The process initiates with the oxidation of unsaturated lipids, generating reactive species that propagate damage throughout the food matrix, leading to the development of off-flavors, deterioration of color and texture, degradation of essential nutrients, and formation of potentially harmful compounds [4] [28]. Protein co-oxidation represents a particularly significant consequence, as lipid-derived radicals and carbonyl compounds modify protein structure, impair digestibility, and diminish technological functionality [4].
Future research should prioritize several key areas to advance understanding and control of lipid oxidation in food systems. The application of advanced omics technologies, particularly lipidomics and proteomics, will provide unprecedented molecular-level insight into oxidative pathways and their specific targets [30] [31]. Elucidation of the relationship between oxidative modification of specific lipid classes and their functional consequences remains a critical knowledge gap [30] [31]. Additionally, research should focus on the development of integrated antioxidant strategies that combine natural antioxidants with physical barrier technologies and processing modifications to maximize protective effects [28] [5]. Understanding the fate and biological effects of lipid oxidation products during digestion and absorption represents another crucial research direction with significant implications for human health [28]. As food formulations increasingly incorporate polyunsaturated lipids for their health benefits, addressing their inherent oxidative instability through targeted stabilization approaches will remain a persistent challenge for food researchers and manufacturers alike.
Analytical Techniques for Lipid Oxidation: From Traditional Assays to Advanced Lipidomics
Lipid oxidation is a fundamental chemical process that leads to the deterioration of fats and oils in food products, resulting in rancidity, loss of nutritional value, and the formation of potentially harmful compounds [3]. This oxidative degradation occurs through a series of complex reactions initiated by the exposure of unsaturated lipids to oxygen, light, and heat [32]. The analysis of primary oxidation products is crucial for assessing the initial stages of lipid oxidation before the development of noticeable rancidity, enabling researchers and food manufacturers to implement preventive measures and maintain product quality.
Within the broader context of lipid oxidation mechanisms and food rancidity research, the measurement of primary oxidation products provides critical insights into the early oxidative stability of lipid-containing systems. Two principal analytical methods have emerged as cornerstone techniques for quantifying primary oxidation: Peroxide Value (PV) and Conjugated Dienes (CD) analysis [33] [32]. These methods target the initial products of oxidation, specifically hydroperoxides and conjugated diene structures, which form when polyunsaturated fatty acids undergo oxidation [3]. Understanding the relationship between these analytical approaches and their application across different lipid systems is essential for comprehensive oxidation assessment in food research and drug development.
Theoretical Foundations of Primary Oxidation
The Lipid Oxidation Mechanism
Lipid oxidation proceeds through a well-established three-stage mechanism of initiation, propagation, and termination [5] [3]. During the initiation phase, free radicals form through the abstraction of hydrogen atoms from bis-allylic methylene groups in polyunsaturated fatty acids, facilitated by factors such as heat, light, or metal catalysts [32] [3]. This process generates lipid radicals (L•) that subsequently react with molecular oxygen in the propagation phase to form lipid peroxyl radicals (LOO•) [3]. These reactive intermediates then abstract hydrogen from adjacent fatty acid molecules, forming lipid hydroperoxides (LOOH) and propagating new lipid radicals in an autocatalytic chain reaction [5].
The termination phase occurs when radical species combine to form non-radical products, effectively concluding the chain reaction [3]. Hydroperoxides, as the primary products of this process, are relatively unstable and decompose further to yield secondary oxidation products including aldehydes, ketones, alcohols, and hydrocarbons, which are responsible for the characteristic off-flavors and odors associated with rancidity [5] [32]. The chemical reactions governing lipid auto-oxidation are illustrated in Figure 1, which maps the complete pathway from initiation through termination.
Figure 1. Lipid auto-oxidation pathway. The three-phase mechanism shows the progression from initiation through propagation to termination, highlighting the formation of primary oxidation products (hydroperoxides) and their decomposition to secondary oxidation products.
Structural Basis of Conjugated Diene Formation
The formation of conjugated dienes represents a structural rearrangement in polyunsaturated fatty acids during the initial stages of oxidation. In linoleic acid (C18:2), oxidation typically initiates at the bis-allylic methylene group at carbon 11, forming a pentadienyl radical that undergoes electron delocalization across five carbon atoms [3]. This delocalization stabilizes the radical intermediate and leads to the formation of conjugated diene structures in the resulting hydroperoxides [3]. Specifically, linoleic acid oxidation produces approximately equal amounts of 9- and 13-hydroperoxides, both containing conjugated diene systems that exhibit characteristic strong absorption at 234 nm [33].
The degree of conjugation formation varies significantly among different fatty acid systems based on their unsaturation pattern. Monounsaturated fatty acids like oleic acid form non-conjugated hydroperoxides that do not absorb significantly at 234 nm, while polyunsaturated fatty acids with three or more double bonds can form complex mixtures of conjugated and non-conjugated oxidation products [33]. This fundamental structural difference has important implications for the application of conjugated diene measurements across different oil types and highlights the complementary nature of PV and CD analysis in comprehensive oxidation assessment.
Analytical Methods for Peroxide Value Determination
Principles and Chemical Basis
The peroxide value (PV) quantifies the concentration of peroxides and hydroperoxides in a lipid sample, expressed as milliequivalents of peroxide oxygen per kilogram of fat or oil (meq O₂/kg) [34] [35]. The fundamental principle underlying PV determination involves the oxidation of iodide ions by hydroperoxides in an acidic environment, liberating iodine according to the reaction:
ROOH + 2H⁺ + 2I⁻ → ROH + H₂O + I₂ [34]
The liberated iodine is then quantified by titration with sodium thiosulfate using starch as an indicator:
I₂ + 2Na₂S₂O₃ → 2NaI + Na₂S₄O₆ [34]
The transition from a blue-black starch-iodine complex to colorless indicates the titration endpoint, with the amount of sodium thiosulfate consumed being directly proportional to the peroxide concentration in the sample [34] [35]. The acidic conditions in the reaction medium prevent the formation of hypoiodite, which could otherwise interfere with the accuracy of the determination [35].
Standardized Methodologies and Protocols
The American Oil Chemists' Society (AOCS) Official Method Cd 8-53 represents the internationally recognized standard procedure for PV determination [33] [36]. The following protocol details the experimental workflow for this reference method:
Reagents Required:
- Glacial acetic acid-chloroform solution (3:2 v/v)
- Saturated potassium iodide (KI) solution
- Sodium thiosulfate (Na₂S₂O₃) solution (0.01 N or 0.002 N, standardized)
- Starch indicator solution (1%)
- Deionized water
Experimental Procedure:
- Sample Preparation: Precisely weigh 5.00 ± 0.05 g of oil or fat sample into a 250 mL glass-stoppered conical flask. For samples with expected high PV (>10 meq/kg), reduce the sample weight accordingly.
Dissolution: Add 30 mL of the acetic acid-chloroform solution (3:2) and swirl gently to dissolve the sample completely.
Iodide Addition: Pipette 0.5 mL of saturated KI solution into the mixture.
Reaction Incubation: Stopper the flask immediately and swirl for exactly 1 minute, then place it in a dark environment for 5 minutes to allow complete reaction while minimizing photo-induced decomposition of peroxides.
Titration: Remove the flask from the dark and add 30 mL of deionized water. Titrate immediately with standardized sodium thiosulfate solution (0.01 N for medium-high PV samples, 0.002 N for low PV samples) while vigorously shaking, until the yellow iodine color almost disappears.
Endpoint Determination: Add 0.5 mL of starch indicator solution and continue titration until the blue color completely disappears, indicating the endpoint.
Blank Determination: Conduct a blank titration using the same reagents without the oil sample.
Calculation: Peroxide Value (meq O₂/kg) = [(S - B) × N × 1000] / W
Where:
- S = Volume of Na₂S₂O₃ used for sample (mL)
- B = Volume of Na₂S₂O₃ used for blank (mL)
- N = Normality of Na₂S₂O₃ solution
- W = Sample weight (g)
Advanced and Alternative Methodologies
While titration remains the standard reference method, several advanced techniques have been developed to address limitations in throughput, sensitivity, and operational safety:
Spectrophotometric Methods: Ferric thiocyanate and similar spectrophotometric assays offer improved sensitivity for low peroxide concentrations, operating on the principle of hydroperoxide oxidation of ferrous (Fe²⁺) to ferric (Fe³⁺) ions, which then form colored complexes with thiocyanate that can be measured at 500-510 nm [32].
Fourier-Transform Infrared (FTIR) Spectroscopy: FTIR techniques enable rapid, non-destructive PV determination by quantifying the absorption bands associated with hydroperoxide functional groups, particularly the O-H stretching vibration at approximately 3500 cm⁻¹ [36]. This approach eliminates the need for chemical reagents and enables high-throughput analysis.
Near-Infrared (NIR) Spectroscopy: NIR spectroscopy coupled with multivariate calibration models (PLS, ridge regression, LASSO) has demonstrated strong correlation with reference titration methods (RMSEP = 1.80-2.50 meq O₂/kg) across diverse oil types including sunflower, olive, and canola oils [36]. This method utilizes spectral information in the 4000-12,500 cm⁻¹ range and benefits from minimal sample preparation.
Table 1: Comparison of Primary Methods for Peroxide Value Determination
| Method | Principle | Detection Limit | Key Advantages | Key Limitations |
|---|---|---|---|---|
| Titration (AOCS Cd 8-53) | Iodometric titration | ~0.1 meq/kg | Official reference method; established accuracy | Time-consuming; chemical waste; requires skilled personnel |
| Spectrophotometric | Colorimetric detection of Fe³⁺-SCN complex | ~0.05 meq/kg | Higher sensitivity; suitable for low PV samples | Interference from colored compounds; requires lipid extraction |
| FTIR | Hydroperoxide O-H stretching at ~3500 cm⁻¹ | ~0.5 meq/kg | Rapid; non-destructive; no chemicals | Requires calibration models; instrument cost |
| NIR | Multivariate calibration on NIR spectra | ~1.0 meq/kg | Fast (<5 minutes); minimal sample prep | Complex calibration; model transfer challenges |
Analytical Methods for Conjugated Dienes Determination
Principles and Structural Basis
The conjugated dienes (CD) method quantifies the early stages of lipid oxidation by measuring the ultraviolet absorption of conjugated diene structures formed during the oxidation of polyunsaturated fatty acids [33] [3]. When linoleic acid and other polyunsaturated fatty acids undergo oxidation, the rearrangement of double bonds creates conjugated diene systems (-CH=CH-CH=CH-) that exhibit characteristic strong absorption at 234 nm due to π-π* electron transitions [33]. The fundamental principle underlying this method is the direct correlation between the concentration of these conjugated diene structures and the extent of primary oxidation in the lipid sample.
The molar absorptivity of conjugated diene hydroperoxides varies slightly depending on the specific fatty acid composition and the position of the hydroperoxide group. For pure methyl linoleate hydroperoxides, the molar absorptivity at 234 nm is approximately 26,000 L·mol⁻¹·cm⁻¹, while complex oil matrices may exhibit variations based on their specific fatty acid profile and the relative proportions of different hydroperoxide isomers [33]. This relationship enables the quantitative assessment of primary oxidation without chemical reactions or derivatization steps.
Standardized Methodology and Protocol
The following protocol details the standardized experimental workflow for conjugated dienes determination in lipid samples:
Reagents and Equipment:
- Isooctane or cyclohexane (UV-spectroscopic grade)
- Absolute ethanol or methanol
- Reference oil sample (unoxidized)
- UV-Visible spectrophotometer with 1 cm quartz cuvettes
- Volumetric flasks (10-25 mL capacity)
- Analytical balance
Experimental Procedure:
- Sample Preparation: Precisely weigh 0.01-0.05 g of oil sample into a 25 mL volumetric flask. For highly oxidized samples, reduce the sample mass accordingly to maintain absorbance values within the linear range (0.1-1.0 AU).
Dissolution: Dilute to volume with isooctane or cyclohexane and mix thoroughly to ensure complete dissolution. For samples insoluble in non-polar solvents, use ethanol or ethanol:isopropanol mixtures (1:1 v/v).
Spectrophotometric Measurement: Transfer the solution to a 1 cm quartz cuvette and measure the absorbance against a solvent blank at 234 nm. Ensure the spectrophotometer is properly zeroed with the pure solvent.
Background Correction: For samples with significant background absorption, measure additional absorbance at 270 nm or 300 nm to correct for non-specific absorption and scattering. Some methodologies also recommend scanning from 200-300 nm to confirm the characteristic conjugated diene peak at 234 nm.
Blank Measurement: Prepare and measure a reference oil sample (unoxidized) of the same type to establish baseline conjugation levels.
Calculation: Conjugated Dienes (mmol/L) = (A × M) / (ε × b × m)
Where:
- A = Corrected absorbance at 234 nm
- M = Molecular weight of the oil (approximately 880 g/mol for most triglycerides)
- ε = Molar absorptivity (typically 25,000-26,000 L·mol⁻¹·cm⁻¹ for mixed hydroperoxides)
- b = Pathlength of cuvette (cm)
- m = Mass of sample (g)
For direct comparison between samples, results are often expressed as specific extinction coefficients: K₂₃₄ = A / (c × d)
Where:
- c = Concentration of oil solution (g/L)
- d = Pathlength (cm)
Methodological Considerations and Limitations
The conjugated dienes method exhibits several important limitations that researchers must consider during experimental design and data interpretation. The relationship between CD values and actual hydroperoxide concentration varies significantly with fatty acid composition, as oils high in oleic acid (e.g., high-oleic sunflower oil) produce substantial amounts of non-conjugated hydroperoxides that are not detected at 234 nm [33]. This compositional dependency necessitates careful calibration when comparing different oil types.
Additionally, secondary oxidation products with conjugated systems may also absorb in the 230-240 nm region, potentially leading to overestimation of primary oxidation products in advanced oxidation stages. The presence of natural pigments, additives, or other UV-absorbing compounds in complex food matrices can interfere with accurate measurement, requiring appropriate background correction or sample purification steps such as solid-phase extraction before analysis.
Comparative Analysis and Method Selection
Correlation Between PV and CD Methods
The relationship between peroxide value and conjugated dienes measurements exhibits significant dependence on the fatty acid composition of the lipid system. Research by Marmesat et al. demonstrated strong linear correlations between PV and CD in methyl linoleate (R² = 0.9988), conventional sunflower oil (R² = 0.9991), and high-oleic sunflower oil (R² = 0.9977) [33]. However, the slopes of these correlations differed substantially: 0.0974 for methyl linoleate, 0.0854 for conventional sunflower oil, and 0.0503 for high-oleic sunflower oil [33]. These differences reflect the varying proportions of conjugated versus non-conjugated hydroperoxides formed during the oxidation of different fatty acid systems.
The decreasing slope with increasing oleic acid content indicates that a significant portion of hydroperoxides formed in high-oleic oils are non-conjugated structures that escape detection by UV absorption at 234 nm [33]. This fundamental relationship highlights that conjugated dienes measurements primarily reflect the oxidation products of linoleic and linolenic acids, while peroxide values capture the total hydroperoxide content regardless of conjugation status. Consequently, the CD method provides a more specific measurement of oxidation in polyunsaturated fatty acids, while PV offers a more comprehensive assessment of overall primary oxidation across diverse fatty acid compositions.
Interpretation Guidelines and Quality Thresholds
Interpretation of PV and CD results requires understanding of established quality thresholds and their relationship to sensory properties. Fresh, high-quality oils typically exhibit PV below 5 meq/kg, while PV between 10-20 meq/kg indicates significant oxidation that may approach the threshold for rancidity detection in some oil types [34]. PV exceeding 30-40 meq/kg generally corresponds to noticeable rancid flavors and represents the upper limit for edible acceptability [35].
For conjugated dienes, the specific extinction coefficient K₂₃₄ provides a relative measure of primary oxidation, with values below 0.5 typically indicating minimal oxidation in vegetable oils. The ratio between PV and CD values can offer insights into the fatty acid specificity of the oxidation process, with lower ratios suggesting greater contribution from non-conjugated hydroperoxides characteristic of monounsaturated fatty acid oxidation.
Table 2: Quality Assessment Guidelines Based on Primary Oxidation Parameters
| Quality Parameter | Excellent Quality | Good Quality | Marginal Quality | Poor Quality/Rancid |
|---|---|---|---|---|
| Peroxide Value (meq O₂/kg) | <2.0 | 2.0-5.0 | 5.0-10.0 | >10.0-20.0 |
| Sensory Correlation | No off-flavors | Minimal detection by trained panelists | Noticeable to trained panelists | Clearly noticeable rancidity |
| Typical CD (K₂₃₄) | <0.15 | 0.15-0.30 | 0.30-0.50 | >0.50 |
| Recommended Action | Acceptable for all uses | Limited shelf-life remaining | Immediate use recommended | Not recommended for consumption |
Application Across Different Matrices
The complementary application of PV and CD methods varies significantly across different food matrices and research objectives. For plant oils rich in polyunsaturated fatty acids (e.g., conventional sunflower oil, soybean oil), both methods provide valuable and correlated information about early oxidation stages [33]. In animal fats and high-oleic oils, PV determination offers more comprehensive assessment due to the prevalence of non-conjugated hydroperoxides [33].
In complex food systems such as emulsions, the interfacial composition significantly influences oxidation pathways, with protein-stabilized emulsions exhibiting different radical initiation mechanisms compared to surfactant-stabilized systems [37]. In these complex matrices, PV determination following lipid extraction provides more reliable assessment of oxidation compared to CD measurements, which can be complicated by light scattering and interference from other UV-absorbing components. For meat and meat products, PV serves as a more appropriate indicator of early oxidation, while secondary oxidation products (e.g., TBARS) often provide better correlation with sensory degradation [32].
Research Applications and Advanced Method Integration
The Researcher's Toolkit: Essential Reagents and Materials
Successful implementation of primary oxidation analysis requires specific research reagents and specialized materials. The following table details essential components for establishing robust PV and CD analytical capabilities:
Table 3: Essential Research Reagents and Materials for Primary Oxidation Analysis
| Reagent/Material | Specification | Primary Function | Method Applicability |
|---|---|---|---|
| Glacial acetic acid | ACS grade, low in reducible oxides | Reaction medium for iodometric titration | PV (Titration) |
| Chloroform | Stabilized with amylene, ACS grade | Lipid solvent for titration medium | PV (Titration) |
| Potassium iodide | ACS grade, ≤0.001% heavy metals | Iodine liberation from hydroperoxides | PV (Titration) |
| Sodium thiosulfate | ACS grade, certified for standardization | Titrant for liberated iodine quantification | PV (Titration) |
| Solvents (UV-spectroscopic) | Isooctane, cyclohexane, or hexane (UV-cutoff <220 nm) | Sample dissolution for UV analysis | CD |
| Reference standards | Methyl linoleate hydroperoxides, purified | Calibration and method validation | PV, CD |
| Antioxidants | BHT, propyl gallate (10-100 ppm) | Prevention of oxidation during analysis | Sample preparation |
| Inert atmosphere | Nitrogen or argon (high purity) | Oxygen exclusion during sample handling | Sample preparation |
Experimental Design Considerations
Optimizing experimental design for primary oxidation analysis requires careful consideration of several critical factors. Sample handling and preparation must minimize artificial oxidation through inert atmosphere (N₂ or Ar) blanketting, temperature control (≤40°C), and limited light exposure [33]. The inclusion of appropriate antioxidant preservatives (e.g., BHT at 50 ppm) during lipid extraction prevents artefactual oxidation, particularly in highly unsaturated samples [32].
Temporal analysis represents another crucial consideration, as the transient nature of hydroperoxides means that single-timepoint measurements provide limited insight into oxidation kinetics [34] [32]. Comprehensive assessment requires monitoring the evolution of both primary and secondary oxidation products throughout the oxidation process, as illustrated in Figure 2, which maps the analytical decision pathway for method selection based on research objectives and sample characteristics.
Figure 2. Analytical method selection workflow. This decision tree guides researchers in selecting appropriate primary oxidation methods based on sample characteristics and research objectives, emphasizing the complementary nature of PV and CD analysis.
Method validation should include assessment of linearity, precision, accuracy, and limit of quantification using appropriate reference materials. For PV titration, standardization against potassium iodate or hydrogen peroxide solutions verifies method accuracy, while purified methyl linoleate hydroperoxides serve as optimal calibration standards for both PV and CD methods [33]. Regular participation in proficiency testing programs and inclusion of control samples in each analytical batch ensures ongoing method performance verification.
Integration in Comprehensive Oxidation Assessment
Within broader lipid oxidation research, PV and CD analysis form the foundation of a comprehensive analytical strategy that should include secondary oxidation measurements and sensory evaluation. The evolving relationship between primary and secondary oxidation products throughout the oxidation process necessitates this integrated approach, as the decomposition of hydroperoxides to aldehydes and ketones fundamentally alters the analytical landscape [5] [32].
Advanced research applications increasingly combine traditional wet chemical methods with spectroscopic techniques to establish predictive models for oxidative stability. Near-infrared spectroscopy coupled with multivariate calibration has demonstrated strong potential for rapid PV determination (RMSEP = 1.80-2.50 meq O₂/kg) across diverse oil types [36]. Similarly, emerging technologies such as NMR spectroscopy, electron spin resonance, and chemiluminescence offer complementary approaches for specific research applications where traditional methods face limitations.
The integration of primary oxidation analysis with protein oxidation assessment represents another advancing research frontier, as lipid oxidation products initiate and propagate protein oxidation through co-oxidation reactions, particularly in protein-stabilized emulsions and muscle foods [3] [37]. This intersection between lipid and protein oxidation mechanisms underscores the importance of PV and CD analysis within comprehensive food quality and stability research frameworks.
Lipid oxidation is a major cause of quality deterioration in fat-containing foods, leading to rancidity, nutrient loss, and the formation of potentially harmful compounds. While primary oxidation produces hydroperoxides, secondary oxidation yields carbonyl compounds, including aldehydes and ketones, which are largely responsible for the undesirable flavors and odors associated with rancid foods. Accurate assessment of these secondary products is crucial for evaluating food quality, shelf-life, and safety. Two widely employed methods for quantifying secondary lipid oxidation are the Thiobarbituric Acid Reactive Substances (TBARS) assay and the p-Anisidine Value (AnV) test. These methods target different classes of secondary oxidation products and provide complementary information about the oxidative state of lipids. Within the broader context of lipid oxidation research, understanding the mechanisms, applications, and limitations of these analytical techniques is fundamental for food scientists, researchers, and industry professionals dedicated to controlling rancidity and improving product stability.
This technical guide provides an in-depth examination of the TBARS and p-Anisidine Value methods. It details their underlying principles, standard protocols, and key applications across various food matrices. The document also presents a direct comparative analysis and discusses the integration of these tests within a comprehensive lipid oxidation evaluation strategy, providing essential knowledge for objective interpretation of analytical data in both research and quality control settings.
Core Principles and Methodologies
The p-Anisidine Value (AnV) Method
The p-Anisidine Value (AnV) is a specific measure of secondary lipid oxidation, primarily targeting aldehydes generated during the advanced stages of oxidation, particularly 2-alkenals and 2,4-alkadienals [38]. These unsaturated aldehydes contribute significantly to the rancid odors and flavors in oxidized oils and fats. The fundamental principle of this method involves the reaction of p-anisidine reagent with the carbonyl groups of these aldehydes in a solution of fat or oil dissolved in iso-octane and glacial acetic acid [38] [39]. This reaction forms yellowish Schiff's base products that absorb light strongly at a wavelength of 350 nm [38]. By convention, the p-Anisidine Value is defined as 100 times the absorbance measured at 350 nm in a 1 cm cuvette of a solution resulting from the reaction of 1 g of fat in 100 mL of solvent with the p-anisidine reagent [38]. A higher AnV indicates a greater concentration of secondary oxidation products and thus a more advanced state of lipid deterioration.
A significant application of the AnV is its use in calculating the TOTOX (Total Oxidation) Value, an empirical parameter that attempts to capture the overall oxidation status by accounting for both primary and secondary oxidation products. The TOTOX value is calculated as TOTOX = 2PV + AnV, where PV is the Peroxide Value [38]. This combined value is particularly useful for evaluating the "oxidative history" of an oil, as it acknowledges that hydroperoxides (measured by PV) are transient and decompose into the aldehydes measured by AnV [40] [38]. The AnV test is especially valuable for assessing oils that have been subjected to heat stress, such as frying oils, and for evaluating refined oils where initial high peroxide levels may have decomposed during deodorization [38].
The Thiobarbituric Acid Reactive Substances (TBARS) Assay
The Thiobarbituric Acid Reactive Substances (TBARS) assay is a widely used method for assessing lipid oxidation, particularly in meat, fish, and other animal-based products [41]. The test measures a range of carbonyl compounds, notably malondialdehyde (MDA), a predominant dialdehyde formed from the oxidation of polyunsaturated fatty acids with three or more double bonds [41]. The principle of the assay relies on the reaction of thiobarbituric acid (TBA) with MDA and other secondary oxidation products (like 2-alkenals and 2,4-alkadienals) under acidic conditions and elevated temperature [41]. This reaction produces a pinkish-red chromogen that can be measured by its absorbance in the 530-540 nm range using a spectrophotometer [41].
The results are typically expressed as mg of MDA per kg of sample or as "TBARS number" [41]. It is critical to note that the test is not specific to MDA alone; it quantifies a group of TBA-reactive substances, hence the name TBARS [41]. This lack of specificity can be a source of interference, as other food constituents, including sugars, acids, and oxidized proteins, can also react with TBA to produce chromogens, potentially leading to overestimation of oxidation levels [41]. For this reason, the TBARS assay is considered most reliable for comparative purposes within the same type of product. Foods with a TBARS value exceeding 1–2 μmol MDA equivalent per gram of fat are generally considered to have a rancid flavor [41]. The method is commonly applied via direct extraction or a distillation procedure, with the latter being preferred for cooked, high-fat, or complex samples to minimize matrix interference and turbidity [41].
Comparative Analysis of TBARS and p-Anisidine Value
While both TBARS and p-Anisidine Value are instrumental in quantifying secondary lipid oxidation, they differ in their specific targets, typical applications, and limitations. The table below provides a structured comparison to guide method selection.
Table 1: Comparative Analysis of TBARS and p-Anisidine Value Methods
| Feature | p-Anisidine Value (AnV) | Thiobarbituric Acid (TBARS) |
|---|---|---|
| Primary Target | Unsaturated aldehydes (2-alkenals, 2,4-alkadienals) [38] | Malondialdehyde (MDA) and other TBA-reactive substances (e.g., 2-alkenals, 2,4-alkadienals) [41] |
| Measured Wavelength | 350 nm [38] [39] | 530-540 nm [41] |
| Primary Application Matrix | Fats, oils, and high-lipid products [40] [38] | Meat, fish, and animal-based products; low-fat samples [41] [5] |
| Key Output/Unit | Anisidine Value (AnV), dimensionless [38] | mg MDA/kg sample [41] |
| Main Advantages | Sensitive to unsaturated aldehydes that correlate well with rancid flavors; used with PV for TOTOX value [38] | Useful for a wide range of sample types; particularly effective for meat and fish products [41] [5] |
| Key Limitations | Not suitable for highly colored oils; reagent (p-anisidine) is highly toxic [38] | Not specific to MDA; susceptible to interference from other food components (sugars, proteins) [41] |
Detailed Experimental Protocols
Protocol for Determining p-Anisidine Value
The following protocol is adapted from standard methods (AOCS Cd 18-90) and bio-protocol resources [39].
Table 2: Key Reagents and Equipment for p-Anisidine Value Analysis
| Item | Specification / Function |
|---|---|
| p-Anisidine Reagent | 0.25% (w/v) in glacial acetic acid. Note: p-Anisidine is highly toxic; use appropriate personal protective equipment (PPE) and handle in a fume hood. [38] [39] |
| Solvent | Isooctane (2,2,4-Trimethylpentane), carbonyl-free [39] |
| Acetic Acid | Glacial acetic acid (99+%) [39] |
| Volumetric Flasks | 25 mL and 200 mL capacity |
| Test Tubes | For reaction and measurement |
| Spectrophotometer | UV-Vis capable of measuring absorbance at 350 nm [39] |
| Cuvettes | 1 cm pathlength, quartz or disposable acrylic |
Procedure:
- Sample Preparation: Dry the oil sample by adding anhydrous sodium sulfate to remove residual water. Filter through a 0.45 µm nylon filter to remove the solid desiccant [39].
- Preparation of Oil Solution: Accurately weigh about 0.5 g of the filtered oil into a 25 mL volumetric flask. Dilute to the mark with isooctane and mix well [39].
- Reaction:
- Pipette 5.0 mL of the oil solution into a test tube.
- Add 1.0 mL of the p-anisidine reagent and mix vigorously.
- Let the reaction proceed for exactly 10 minutes at room temperature [39].
- Blank Preparation: In parallel, prepare a blank by mixing 5.0 mL of the same oil solution with 1.0 mL of glacial acetic acid (without p-anisidine).
- Absorbance Measurement: After exactly 10 minutes, measure the absorbance of the reacted solution at 350 nm against a blank of pure isooctane in the reference cell. Then, measure the absorbance of the blank solution (oil + acetic acid) at the same wavelength against the same reference [38] [39].
- Calculation: The p-Anisidine Value (AnV) is calculated using the following formula:
AnV = (25 × (1.2As - Ab)) / W
Where:
A_s= Absorbance of the test solution (oil + p-anisidine reagent)A_b= Absorbance of the blank solution (oil + acetic acid)W= Mass of the sample in grams [38].
Protocol for Determining TBARS (Distillation Method for Meat/Fish)
This protocol is suitable for cooked meats, high-fat samples, or products where turbidity is an issue [41].
Table 3: Key Reagents and Equipment for TBARS Analysis
| Item | Specification / Function |
|---|---|
| TCA Solution | 7.5% (w/v) Trichloroacetic Acid, used for extraction and protein precipitation. |
| TBA Reagent | Thiobarbituric acid solution, typically 0.02M in water or a TCA/water mixture. |
| Standard | 1,1,3,3-Tetraethoxypropane (TEP), which hydrolyzes to malondialdehyde (MDA). |
| Distillation Apparatus | Glassware for steam or simple distillation. |
| Water Bath | Capable of maintaining 100°C for incubation. |
| Spectrophotometer | Visible light capable of measuring absorbance at 532-540 nm. |
Procedure:
- Sample Homogenization: Homogenize a representative sample (e.g., 10 g) with a 7.5% TCA solution (e.g., 50 mL) to precipitate proteins and extract MDA.
- Centrifugation/Filtration: Centrifuge the homogenate or filter it to obtain a clear supernatant.
- Distillation (for complex matrices): Transfer an aliquot of the supernatant to a distillation apparatus. Distill at a controlled rate and collect a predetermined volume of distillate. Note: Standards (TEP) must be carried through the same distillation process to account for recovery losses [41].
- Color Development: Pipette a portion (e.g., 5 mL) of the distillate (or clear extract for simple samples) into a test tube. Add an equal volume (e.g., 5 mL) of TBA reagent. Cap the tubes and mix well.
- Incubation: Place the test tubes in a boiling water bath for 35 minutes to develop the pink color [41].
- Cooling and Measurement: Remove the tubes and cool them under running tap water or in an ice bath. Measure the absorbance of the solution at 532 nm or 540 nm against a blank prepared with TCA and TBA reagent.
- Calibration and Calculation: Prepare a standard curve using known concentrations of MDA generated from TEP. Express the results as mg of MDA per kg of sample based on the standard curve [41].
Integration in Lipid Oxidation Research and Data Interpretation
The Role of TBARS and AnV in a Comprehensive Analytical Strategy
In food rancidity research, TBARS and p-Anisidine Value are most powerful when used as part of an integrated analytical strategy rather than as standalone tests. Lipid oxidation is a dynamic process, and the concentrations of primary and secondary products change over time. The p-Anisidine Value, which measures secondary carbonyls, is often used in conjunction with the Peroxide Value (PV), which measures primary hydroperoxides [38] [5]. As shown in the conceptual diagram below, PV rises and falls during oxidation, while AnV and TBARS, which measure secondary products, generally increase as oxidation progresses and stabilize or continue to rise in the termination phase [5]. The TBARS assay follows a similar trajectory to AnV but is applied to different food matrices.
Figure 1: Relationship between lipid oxidation stages and analytical markers. Peroxide Value (PV) peaks during propagation, while AnV and TBARS, measuring secondary products, increase and persist into the termination phase.
The Scientist's Toolkit: Essential Research Reagents
Successful execution of TBARS and AnV assays requires specific, high-purity reagents. The following table details essential materials and their critical functions within the experimental workflows.
Table 4: Essential Research Reagent Solutions for Secondary Oxidation Analysis
| Reagent / Material | Function in Analysis | Key Considerations |
|---|---|---|
| p-Anisidine | Reacts with unsaturated aldehydes (2-alkenals, 2,4-alkadienals) to form a colored product measurable at 350 nm [38] [39]. | Highly toxic. Handle with extreme care using appropriate PPE (gloves, safety glasses) and in a well-ventilated fume hood. Requires proper hazardous waste disposal [38]. |
| Thiobarbituric Acid (TBA) | Reacts with malondialdehyde (MDA) and other carbonyls to form a pink chromogen measurable at 532-540 nm [41]. | Susceptible to interference; not specific to MDA. The reaction is influenced by time, temperature, and pH, requiring strict protocol adherence [41]. |
| Trichloroacetic Acid (TCA) | Used in TBARS assay to precipitate proteins, extract MDA, and acidify the medium to facilitate the TBA reaction [41]. | Corrosive. Causes severe skin burns and eye damage. |
| 1,1,3,3-Tetraethoxypropane (TEP) | Stable precursor that hydrolyzes to malondialdehyde (MDA), used for preparing the standard calibration curve in the TBARS assay [41]. | Essential for quantifying results as mg MDA/kg. Must be carried through the entire analytical process (e.g., distillation) if applicable [41]. |
| Isooctane | Solvent used to dissolve the oil/fat sample in the p-Anisidine Value method [38] [39]. | Must be carbonyl-free to prevent interference with the absorbance measurement. |
| Glacial Acetic Acid | Solvent for p-anisidine reagent and reaction medium for the color development [38] [39]. | Corrosive and has a pungent odor. Use in a fume hood. |
The TBARS and p-Anisidine Value methods are cornerstone analytical techniques for quantifying secondary lipid oxidation products, each with distinct applications and strengths. The p-Anisidine Value is particularly valuable for tracking the history of oxidation in fats and oils, especially when used with the Peroxide Value to calculate the TOTOX value. The TBARS assay, despite its lack of absolute specificity, remains a highly sensitive and widely adopted method for evaluating oxidative rancidity in meat, fish, and other complex food systems.
A thorough understanding of the underlying chemical principles, standardized protocols, and inherent limitations of both methods is crucial for generating reliable and interpretable data. The choice between them should be guided by the specific food matrix, the type of information required, and the available laboratory resources. Furthermore, these methods should not be used in isolation. Integrating them with sensory analysis and other instrumental techniques (e.g., GC-MS for volatile profiling) provides the most comprehensive assessment of lipid oxidation and its impact on food quality and shelf-life. As research advances, the development of more specific, non-toxic, and high-throughput methods will continue to evolve, but TBARS and AnV will remain fundamental tools in the food scientist's arsenal for combating rancidity.
Lipid oxidation is a primary cause of quality deterioration in foods and biological systems, leading to the formation of rancid flavors, loss of nutritional value, and generation of potentially harmful compounds [42] [4]. This complex process involves the reaction of unsaturated lipids with molecular oxygen via multiple pathways, including auto-oxidation (free radical chain reactions), photo-oxidation (singlet oxygen reactions), and enzymatic oxidation [4]. The oxidation of lipids proceeds through distinct stages: initially, hydroperoxides form as primary oxidation products, which then decompose into a wide range of secondary oxidation products, including volatile carbonyls, alcohols, hydrocarbons, and epoxides [4]. Understanding these mechanisms is critically important for stabilizing foods, reducing food costs and losses, and maintaining food safety and quality [31].
Chromatographic profiling techniques provide powerful tools for elucidating lipid oxidation mechanisms by identifying and quantifying both primary and secondary oxidation products. High-Performance Liquid Chromatography coupled with tandem Mass Spectrometry (HPLC-MS/MS) enables direct identification and quantification of labile hydroperoxide isomers, whose positional distribution provides crucial information about the dominant oxidation mechanisms [43]. Meanwhile, Gas Chromatography-Mass Spectrometry (GC-MS) serves as the primary technique for profiling the complex mixtures of volatile compounds formed during the advanced stages of lipid oxidation [44]. Together, these techniques form a complementary analytical framework for comprehensive characterization of lipid oxidation processes, from initial formation of primary oxidation products to the development of secondary volatiles that contribute to rancid aromas and flavors.
HPLC-MS/MS Analysis of Lipid Hydroperoxides
Technical Principles and Isomer Differentiation
HPLC-MS/MS enables the direct analysis of hydroperoxide isomers without derivatization by leveraging characteristic fragmentation patterns that reveal hydroperoxide group positioning [43]. The technique identifies hydroperoxides through α-cleavage fragmentation adjacent to the carbon bearing the hydroperoxide group, producing diagnostic ions that are monitored with high selectivity in Multiple Reaction Monitoring (MRM) mode [43]. This approach can distinguish between radical-initiated oxidation and singlet oxygen oxidation based on the specific isomers detected. For docosahexaenoic acid (DHA), radical oxidation produces hydroperoxide isomers with hydroperoxyl groups at carbons 4, 7, 8, 10, 11, 13, 14, 16, 17, or 20, while singlet oxygen oxidation additionally yields DHA;5OOH and DHA;19OOH isomers [43].
The analytical strategy involves reverse-phase chromatography to separate isomeric hydroperoxides prior to mass spectrometric detection. For esterified DHA hydroperoxides in complex lipid classes like phosphatidylcholine (PC) and triacylglycerol (TG), the method successfully differentiates isomers in biological matrices such as mackerel tissue, demonstrating its applicability to real food systems [43]. This direct analysis approach is particularly valuable because DHA and other polyunsaturated fatty acids are easily oxidized during sample preparation and derivatization procedures typically used in gas chromatographic analyses.
Experimental Protocol for Hydroperoxide Analysis
Table 1: HPLC-MS/MS Parameters for Hydroperoxide Isomer Analysis
| Parameter | Configuration for Phospholipid Hydroperoxides | Configuration for Neutral Lipid Hydroperoxides |
|---|---|---|
| HPLC System | ExionLC system (SCIEX) | ExionLC system (SCIEX) |
| Column | COSMOSIL Packed Column (5C18-MS-II, 5 μm, 2.0 ID × 250 mm) | ACQUITY UPLC HSS C18 Column (100 Å, 1.8 μm, 2.1 × 150 mm) |
| Mobile Phase | Isocratic elution with methanol/water (95:5, v/v) | Binary gradient: solvent A (methanol), solvent B (2-propanol); 0-20 min, 20-100% B |
| Flow Rate | 0.2 mL/min | 0.2 mL/min |
| Column Temperature | 45°C | 45°C |
| Mass Spectrometer | 4000 QTRAP mass spectrometer (SCIEX) | 4000 QTRAP mass spectrometer (SCIEX) |
| Ionization Mode | ESI positive | ESI positive |
| Adduct Formation | Na+ adducts using post-column sodium acetate infusion | Na+ adducts using post-column sodium acetate infusion |
| Detection Mode | Product ion scan & MRM | Product ion scan & MRM |
Table 2: Key Research Reagents for Hydroperoxide Analysis
| Reagent/Chemical | Function/Application | Specific Example |
|---|---|---|
| Diolein (DG 18:1_18:1) | Neutral lipid standard for method development | Used in neutral lipid hydroperoxide analysis [43] |
| 1-Palmitoyl-lysophosphatidylcholine | Phospholipid precursor for synthesis of oxidation substrates | Esterified with DHA to produce PC 16:0/22:6 for oxidation studies [43] |
| Rose Bengal | Photosensitizer for generating singlet oxygen | Added to lipid solutions to induce photo-oxidation [43] |
| Butyl Hydroxytoluene (BHT) | Antioxidant to prevent further oxidation during analysis | Added to samples to arrest oxidation at specific time points [43] |
| N,N'-Dicyclohexylcarbodiimide (DCC) | Coupling reagent for chemical synthesis | Used in esterification of DHA to LPC [43] |
| Dimethylaminopyridine (DMAP) | Acylation catalyst | Accelerates esterification reaction in synthesis of phospholipid substrates [43] |
| Sodium Acetate Solution | Post-column additive to promote sodium adduct formation | Mixed with column eluate to enhance MS detection of lipids as Na+ adducts [43] |
The experimental workflow begins with lipid extraction using modified Folch extraction (chloroform:methanol, 2:1 v/v) [43]. For complex biological samples, solid-phase extraction on aminopropyl cartridges separates neutral lipids (eluted with chloroform/2-propanol, 2:1 v/v) from phospholipids (eluted with methanol) [43]. For model system studies, specific lipid substrates are prepared synthetically - for example, PC 16:0/22:6 is synthesized by esterifying DHA to LPC 16:0/0:0 using DCC and DMAP as coupling reagents in chloroform under nitrogen atmosphere for 28 hours at 40°C [43].
Hydroperoxides are generated experimentally by controlled oxidation of lipid substrates. For singlet oxygen-mediated oxidation, rose bengal is added as a photosensitizer, and the solution is exposed to light [43]. For radical-mediated oxidation, lipids are incubated under controlled temperatures. The resulting hydroperoxide isomers are then separated and identified using the HPLC-MS/MS parameters detailed in Table 1.
Figure 1: Experimental workflow for HPLC-MS/MS analysis of lipid hydroperoxide isomers
GC-MS Analysis of Volatile Oxidation Compounds
Technical Principles and Volatile Profiling
GC-MS enables comprehensive profiling of volatile organic compounds generated during lipid oxidation by combining high-resolution gas chromatographic separation with mass spectrometric identification [44]. This technique is particularly valuable for analyzing secondary lipid oxidation products including aldehydes, ketones, alcohols, hydrocarbons, and furans that contribute to rancid aromas and flavors in oxidized foods [44]. The headspace solid-phase microextraction (HS-SPME) technique is frequently coupled with GC-MS for non-destructive extraction of volatile compounds without solvent use, concentrating analytes through adsorption onto coated fibers [44].
Key volatile markers of lipid oxidation include hexanal, nonanal, and 2-pentylfuran, which show substantial increases during oxidation processes [44]. These compounds serve as sensitive indicators of oxidative rancidity, with their rising concentrations correlating with sensory perception of rancidity. Conversely, compounds like linalool, α-terpineol, d-limonene, and 1-methoxy-nonane typically decrease during oxidation, providing additional diagnostic information about the progression of oxidative deterioration [44]. The technique can detect a wide range of volatile compounds, with one study identifying 65 volatile compounds in fresh infant nutrition packages and 9 newly formed volatile compounds during accelerated oxidation at 45°C for 4 weeks [44].
Experimental Protocol for Volatile Compound Analysis
Table 3: HS-SPME/GC-MS Parameters for Volatile Compound Analysis
| Parameter | Configuration |
|---|---|
| Sample Preparation | 1.00 g sample in 20 mL headspace vial |
| SPME Fiber | 50/30 μm DVB/CAR/PDMS |
| Equilibration Conditions | 50°C for 35 min, shaking every 10 min |
| Extraction Conditions | 70°C for 30 min in headspace |
| Desorption Conditions | GC injector, 260°C for 5 min |
| GC Column | HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm) |
| Carrier Gas | Helium, constant flow 0.8 mL/min |
| Oven Temperature Program | 35°C (hold 3 min), to 65°C at 4°C/min (hold 2 min), to 90°C at 2°C/min (hold 3 min), to 220°C at 5°C/min (hold 2 min) |
| Total Run Time | 56 min |
| Injection Mode | Split (5:1 ratio) |
| Mass Spectrometer | Triple quadrupole MS |
| Ionization Mode | EI, 70 eV |
| Ion Source Temperature | 230°C |
| Mass Range | m/z 50-550 |
| Solvent Delay | 3 min |
Table 4: Key Volatile Markers of Lipid Oxidation
| Volatile Compound | Chemical Class | Trend During Oxidation | Significance |
|---|---|---|---|
| Hexanal | Aldehyde | Substantial increase | Major marker of ω-6 fatty acid oxidation |
| Nonanal | Aldehyde | Substantial increase | Marker of oleic acid oxidation |
| 2-Pentylfuran | Furan | Substantial increase | Secondary oxidation product from linoleic acid |
| 3-Hydroxy-2-methylpyran-4-one | Oxygenated heterocycle | Variable: increases, decreases, then increases | Newly formed during oxidation process |
| Linalool | Alcohol | Downward trend | Degradation of fresh aromas |
| α-Terpineol | Alcohol | Downward trend | Degradation of fresh aromas |
| d-Limonene | Cycloalkene | Downward trend | Degradation of fresh aromas |
| 1-Methoxy-nonane | Ether | Downward trend | Degradation of fresh aromas |
The analytical protocol begins with careful sample preparation, where 1.00 g of sample is placed in a 20 mL headspace vial and sealed with a silicone/Teflon septum cap [44]. The HS-SPME extraction proceeds with vial equilibration at 50°C for 35 minutes in a water bath, with shaking every 10 minutes to facilitate volatile release into the headspace [44]. The SPME fiber is then exposed to the sample headspace for 30 minutes at 70°C to adsorb volatile compounds, followed by thermal desorption in the GC injector port at 260°C for 5 minutes [44].
Chromatographic separation employs a temperature ramp program (detailed in Table 3) designed to resolve complex mixtures of volatile compounds with varying polarities and molecular weights [44]. Compound identification combines retention index matching using the Kovats retention index formula with mass spectral library matching against databases such as the National Institute of Standard and Technology (NIST) database [44]. The retention index calculation uses a series of n-alkane standards (C7-C30) analyzed under identical chromatographic conditions, with the formula: RI = 100n + 100(Tx - Tn)/(Tn+1 - Tn), where Tx is the retention time of the compound, and Tn and Tn+1 are retention times of n-alkanes with n and n+1 carbon atoms, respectively [44].
Figure 2: Experimental workflow for HS-SPME/GC-MS analysis of volatile oxidation compounds
Integrated Applications in Food Rancidity Research
Case Studies in Food Systems
Integrated chromatographic profiling has been successfully applied to diverse food systems to elucidate lipid oxidation mechanisms and track quality deterioration. In mackerel rich in DHA, HPLC-MS/MS analysis revealed that radical oxidation of esterified DHA progresses significantly even during refrigeration, with different transition patterns observed depending on the oxidation mechanism and lipid class [43]. This finding has important implications for storage stability of seafood products and demonstrates the value of direct hydroperoxide isomer analysis for understanding oxidation pathways in complex biological matrices.
In infant nutrition packages, HS-SPME/GC-MS tracking of volatile compounds during accelerated oxidation at 45°C over 4 weeks revealed dynamic changes in the volatile profile [44]. The relative content of volatile substances gradually changed during oxidation, with hexanal, nonanal, and 2-pentylfuran showing substantial increases, while fresh aroma compounds like linalool and d-limonene decreased [44]. Sensory evaluation correlated these chemical changes with progressive quality deterioration from mild bean powder fragrance to severe rancid odor, providing validation for the chemical analyses [44].
Recent research applying lipidomics approaches to watermelon seed kernels has further demonstrated the power of integrated techniques for exploring oxidative rancidity mechanisms and changes in volatile flavors [45]. Such studies highlight how complementary chromatographic techniques can identify oxidation biomarkers and provide mechanistic insights into rancidity development across diverse food matrices.
Implications for Food Quality and Stability
The insights gained from chromatographic profiling of lipid oxidation products have significant implications for food quality, stability, and nutritional safety. Lipid oxidation not only affects sensory qualities but also compromises nutritional value and generates potentially toxic compounds [4] [31]. The free radicals generated during lipid oxidation can induce protein co-oxidation, leading to protein aggregation that significantly affects physicochemical characteristics and biological functions, including digestibility, foaming characteristics, and bioavailability [4]. This co-oxidation phenomenon reduces the edible and storage quality of food and represents an important consideration for food formulators and processors.
Understanding specific oxidation mechanisms enables more targeted stabilization approaches. For instance, identifying singlet oxygen as the dominant oxidation mechanism in a particular food would suggest the use of singlet oxygen quenchers like carotenoids, while dominance of radical oxidation would indicate radical scavengers like tocopherols as more effective antioxidants [43] [4]. This mechanistic approach to food stabilization represents a significant advancement over traditional empirical methods and can lead to more effective preservation strategies for extending shelf-life and maintaining food quality.
Technical Considerations and Method Optimization
Analytical Challenges and Solutions
Several technical challenges require careful consideration when implementing chromatographic profiling methods for lipid oxidation analysis. Hydroperoxide instability during analysis presents a particular challenge, as these primary oxidation products are susceptible to degradation during sample preparation, storage, and analysis [43]. This can be mitigated through careful temperature control, addition of stabilizers like BHT where appropriate, and minimization of analysis time [43]. For esterified hydroperoxides in complex lipids, the development of specific MRM transitions for each isomer is essential for selective detection [43].
In GC-MS analysis, fiber selection for HS-SPME significantly impacts the volatile profile obtained, with divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fibers proving effective for broad ranges of volatile compounds [44]. Optimization of extraction time and temperature is necessary to balance extraction efficiency with potential artifact formation, while regular fiber conditioning and replacement maintain analytical reproducibility [44]. For both techniques, internal standardization with appropriate labeled compounds improves quantification accuracy, though this is more straightforward for GC-MS than for hydroperoxide isomer analysis by HPLC-MS/MS.
Data Interpretation and Correlation
Effective interpretation of chromatographic data requires understanding of lipid oxidation mechanisms and expected product patterns. For example, the distribution of hydroperoxide isomers indicates whether radical or singlet oxygen mechanisms predominate, while specific volatile compound ratios can reveal which polyunsaturated fatty acids are primarily undergoing oxidation [43] [4]. Multivariate statistical analysis of the comprehensive datasets generated by these techniques can identify patterns and correlations not apparent through univariate analysis, supporting more robust conclusions about oxidation pathways and progression.
Correlation between chemical measurements and sensory evaluation provides essential validation for analytical methods, as ultimately the sensory impact of oxidation determines product acceptability [44]. Establishing such correlations enables development of predictive models for shelf-life determination and quality assessment, reducing reliance on time-consuming sensory panels for routine quality control while maintaining alignment with consumer perception.
Lipid oxidation is a dominant cause of quality deterioration in fats and oils, leading to the development of off-flavors, loss of nutritional value, and generation of potentially toxic compounds. This process poses a significant challenge for the food industry and researchers aiming to predict and extend product shelf-life [46]. Oxidation occurs through a complex free radical chain mechanism comprising initiation, propagation, and termination stages, ultimately resulting in a wide range of primary and secondary oxidation products [47] [32]. Monitoring this process is essential for quality control, and while direct chemical methods exist to measure specific oxidation products, they often provide only a snapshot of the current state. Indirect and accelerated methods, such as sensory evaluation and the Rancimat test, are therefore critical tools for comprehensively assessing stability and predicting long-term oxidative behavior [48] [49]. This guide details the principles, protocols, and application of these key methodologies within the broader context of food rancidity research.
Lipid Oxidation: Core Mechanisms and Analysis Framework
The Oxidation Cascade
Understanding the methods for assessing oxidation first requires a grounding in the fundamental mechanisms. Lipid oxidation is a radical-based process that progresses through three key stages, as illustrated in the diagram below.
The process begins with initiation, where an initiator (e.g., heat, light, metal ions) causes a lipid (LH) to lose a hydrogen atom, forming a lipid radical (L•) [47] [46]. During propagation, this radical reacts rapidly with oxygen to form a peroxyl radical (LOO•), which then abstracts a hydrogen from another lipid molecule, yielding a hydroperoxide (LOOH) and a new lipid radical, thus propagating the chain [48]. Hydroperoxides are the primary oxidation products; they are flavorless but unstable and break down into secondary oxidation products, including a wide range of volatile compounds like aldehydes and ketones [32]. These secondary products are responsible for the characteristic rancid odors and flavors. The chain reaction concludes with termination, which occurs when radical species combine to form stable, non-radical products [47].
Analytical Approaches: From Direct Measurement to Accelerated Stability
The analysis of lipid oxidation can be categorized based on the target analyte and the approach.
- Direct Methods: These quantify specific chemical compounds formed during oxidation. Measurement of peroxide value (PV) and conjugated dienes are used to assess primary products, while the p-anisidine value (AnV) and thiobarbituric acid-reactive substances (TBARS) assay target secondary products like aldehydes [48] [32]. Chromatographic techniques can provide detailed profiles of volatile compounds.
- Indirect & Accelerated Methods: These are the focus of this guide. Sensory evaluation is an indirect method that assesses the human perception of the end-result of oxidation (off-flavors) [32]. Accelerated stability tests, like the Rancimat method, force the oxidation of a sample under elevated temperatures to rapidly determine its resistance to oxidation, providing a predictive Oxidative Stability Index (OSI) [48] [49].
Sensory Evaluation: The Human Sensor
Principle and Workflow
Sensory evaluation is a qualitative and quantitative indirect method that relies on human assessors to detect the off-flavors and odors resulting from lipid oxidation. It is a critical tool because the ultimate measure of unacceptability is often consumer perception. The presence of off-flavors, described as "rancid," "painty," or "grassy," indicates that significant oxidation has already occurred [32]. The workflow for a simple sensory test is as follows.
Experimental Protocol for Simple Oil Screening
This protocol outlines a basic screening test suitable for preliminary assessment of edible oils [50].
Materials:
- Sample oil
- Clean glass bowl or cup
- Trained or untrained panelists (a minimum of 5 is recommended for meaningful feedback)
Procedure:
- Ensure the oil sample is at room temperature.
- Pour a few milliliters of oil into a clean glass bowl or cup.
- Olfactory Test: Panelists should gently smell the sample. They are to note any odors that are not fresh, particularly sweet, fermented, or otherwise "off" smells.
- Gustatory Test: Panelists then take a small amount of oil into their mouths without swallowing. They should swirl it in the mouth to assess the taste, noting any rancid, bitter, or other unpleasant flavors.
- Interpretation: A positive identification of off-odors or off-flavors by multiple panelists indicates the oil has undergone significant oxidation and is likely rancid. It is important to note that a lack of flavor does not guarantee the oil is sound, as early-stage oxidation may not be sensorily detectable [50].
Advantages and Limitations in Research
Sensory analysis is invaluable as it directly measures consumer-relevant properties. However, it is subjective, requires careful panel management, and is not sensitive enough to detect the early stages of oxidation, making it a reactive rather than predictive tool [32].
The Rancimat Test: An Accelerated Oxidation Method
Principle and Workflow
The Rancimat method, also known as the Oxidation Stability Index (OSI) test, is an internationally standardized accelerated aging test (e.g., AOCS Cd 12b-92, ISO 6886, EN 14112) [49] [51]. Its principle is based on accelerating the oxidation process by exposing the oil or fat to elevated temperatures (typically between 90–160°C) while a constant stream of air is passed through it [48] [49]. This forced oxidation rapidly degrades the sample. The volatile secondary oxidation products formed—primarily short-chain organic acids like formic and acetic acid—are transported by the air stream into a measuring vessel containing deionized water. The absorption of these acidic compounds increases the electrical conductivity of the water. The instrument continuously monitors this conductivity. The time taken until a sharp increase in conductivity is observed is known as the induction time or OSI, which is a key parameter for predicting shelf-life [49] [51]. The workflow is illustrated below.
Detailed Experimental Protocol
This protocol is adapted from manufacturer guidelines and recent research applications [50] [49].
Materials and Reagents:
- Rancimat instrument (e.g., Metrohm 892 Rancimat)
- Sample reaction vessels and air supply tubes
- Disposable plastic spatulas for weighing
- High-purity deionized water (conductivity < 5 µS/cm)
- Drying oven and desiccator
- Oil or fat sample (ca. 3 g per test)
Procedure:
- Instrument Setup: Turn on the Rancimat and allow the heating block to reach the set temperature (e.g., 110°C is common, but temperatures up to 130°C may be used for faster results). Ensure stability before proceeding.
- Sample Preparation: Using a plastic spatula (to avoid metal ion contamination that can catalyze oxidation), weigh approximately 3 g of the sample directly into a clean, dry reaction vessel. The filling height should not exceed 3.5 cm.
- Assembly: Fill the measuring vessel with deionized water. Correctly position the air supply tube in the reaction vessel and the PTFE transfer cannula in the measuring vessel, ensuring it is not directly aligned with the conductivity electrodes to prevent bubble interference.
- Measurement: Place the reaction vessel into the preheated heating block and connect the air supply. Start the measurement via the instrument software (e.g., StabNet). The air flow (typically 10-20 L/h) will carry volatile compounds into the measuring vessel.
- Data Collection: The software records the conductivity in real-time. The induction time is automatically determined from the resulting curve as the time to the point of maximum change in the conductivity (the inflection point).
- Cleaning: After measurement, cool and dispose of the reaction vessel and air tube. Clean reusable glassware thoroughly in a laboratory dishwasher and dry at 80°C for at least two hours to prevent carry-over of reaction products.
Critical Best Practices:
- Avoid Contamination: Do not use metal spatulas. Ensure all glassware is impeccably clean.
- Ensure Sealing: Check that the green reaction vessel lids provide a tight seal. A leaking seal will cause irreproducible results.
- Proper Alignment: Correct positioning of the air tube and transfer cannula is crucial for obtaining a smooth, evaluable conductivity curve [50].
Key Research Reagent Solutions
Table 1: Essential materials and reagents for the Rancimat test.
| Item | Function/Description | Critical Consideration |
|---|---|---|
| Rancimat Instrument | Dedicated system for OSI determination, with temperature control, air flow, and conductivity measurement. | Must conform to international standards (e.g., EN 14112). Multi-position instruments enable high throughput. |
| Reaction Vessels | Disposable glassware holding the sample during analysis. | Prevents cross-contamination. Maximum fill height (3.5 cm) must be respected. |
| Deionized Water | Absorption solution for volatile acids in the measuring vessel. | Must be high-purity (conductivity <5 µS/cm) to ensure low background and accurate detection. |
| Plastic Spatulas | For weighing and handling samples. | Essential to avoid catalytic contamination from metal ions. |
| Compressed Air Supply | Provides a constant flow of air to oxidize the sample and transfer volatiles. | Requires an in-line filter to remove oil, water, and particles that could cause flow fluctuations. |
Data Interpretation and Correlation with Shelf-Life
Analyzing Rancimat Output
The primary data output from a Rancimat test is a plot of conductivity (µS/cm) versus time (hours). The induction time is the key metric read from this plot, representing the sample's resistance to oxidation under the test conditions. A longer induction time indicates higher oxidative stability. This value can be used for comparative studies, for instance, to evaluate the efficacy of different antioxidants or the impact of various processing techniques on oil stability [48].
Predictive Kinetic Modeling
The power of the Rancimat method lies in its ability to predict shelf-life at ambient conditions. By conducting tests at multiple temperatures, researchers can apply the Arrhenius equation to model the temperature dependence of the oxidation reaction. This allows for the calculation of kinetic parameters, such as activation energy (Ea), and the extrapolation of the Oxidative Stability Index at 20°C (OSI20), which is expressed in months and provides a direct estimate of potential shelf-life [48].
Table 2: Exemplary Rancimat data and kinetic parameters for different olive oils. [48]
| Oil Sample | Extraction Method | OSI at 120°C (h) | OSI at 20°C (Months) | Activation Energy, Ea (kJ/mol) |
|---|---|---|---|---|
| cOO | Conventional 2-phase | ~(Value from Fig 2) | 38.5 (Rancimat) | Calculated from multi-temp data |
| eOO | Expeller from dehydrated olives | ~(Value from Fig 2) | 43.3 (Rancimat) | Calculated from multi-temp data |
| SCOO | Supercritical CO₂ | ~(Value from Fig 2) | 138.6 (Rancimat) | Calculated from multi-temp data |
The data in Table 2 demonstrates a strong correlation between OSI20 and the total phenolic content of the oils, confirming that natural antioxidants significantly enhance oxidative stability and can profoundly extend shelf-life [48].
Within the framework of lipid oxidation research, both sensory evaluation and the Rancimat test provide critical, complementary insights. Sensory analysis serves as an indispensable, though final, arbiter of quality, directly assessing the consumer's experience of rancidity. In contrast, the Rancimat test offers a robust, accelerated, and quantitative predictive tool. Its ability to generate kinetic data for shelf-life modeling makes it an essential technique for research and development, quality control, and formulation science. Used in conjunction with direct chemical analyses, these methods provide a comprehensive toolkit for understanding, monitoring, and ultimately controlling the complex process of lipid oxidation, thereby enabling the production of safer, higher-quality food products with extended shelf lives.
Lipid oxidation is a primary cause of quality deterioration in foods, leading to rancidity, nutrient loss, and the formation of potentially harmful compounds [52]. This complex process involves the autoxidation of unsaturated fatty acids through a free-radical chain reaction mechanism, producing both primary and secondary oxidation products [3]. The selection of appropriate analytical methods is critical for accurately assessing oxidative status in diverse food matrices. Method selection must balance accuracy, sensitivity, and matrix compatibility to generate reliable data for research and quality control purposes.
This technical guide examines the core analytical approaches for monitoring lipid oxidation, with particular emphasis on their applicability to different food systems. The mechanisms of lipid oxidation begin with the formation of free radicals from unsaturated fatty acids upon exposure to initiators like light, heat, or metal catalysts [3]. During the propagation phase, these radicals react with oxygen to form peroxyl radicals, which then abstract hydrogen from other fatty acid molecules, forming hydroperoxides as primary oxidation products [52]. These unstable compounds subsequently decompose into secondary oxidation products including aldehydes, ketones, alcohols, and hydrocarbons, which are responsible for the characteristic off-flavors and aromas associated with rancidity [52] [3].
Core Analytical Methods for Lipid Oxidation Assessment
Analytical methods for assessing lipid oxidation target different stages of the oxidative process, each with distinct advantages, limitations, and matrix compatibilities. These methods can be broadly categorized into those measuring primary oxidation products, those detecting secondary oxidation products, and indirect assessment techniques.
Table 1: Methods for Detecting Primary Lipid Oxidation Products
| Method | Target Analytes | Principle | Typical Food Applications | Key Advantages | Key Limitations |
|---|---|---|---|---|---|
| Peroxide Value (PV) | Hydroperoxides | Titration or spectrophotometric measurement of oxidized iodine | Plant oils, high-fat products | Measures early oxidation; standardized methods | Hydroperoxides unstable; sensitive to oxygen interference [52] |
| Conjugated Diene Analysis | Conjugated dienes | UV absorption at 233-234 nm | Polyunsaturated oils | Rapid; no reagents required; measures early oxidation | Limited to systems with fatty acid conjugation [52] [3] |
| Ferric Thiocyanate | Hydroperoxides | Oxidation of Fe²⁺ to Fe³⁺ followed by complex formation with thiocyanate | Insect-based foods [3] | Simpler than iodometric method | Not suitable for long-term stored meat with decomposing hydroperoxides [3] |
Table 2: Methods for Detecting Secondary Lipid Oxidation Products
| Method | Target Analytes | Principle | Typical Food Applications | Key Advantages | Key Limitations |
|---|---|---|---|---|---|
| Thiobarbituric Acid Reactive Substances (TBARS) | Malondialdehyde (MDA) and other carbonyls | Reaction with TBA to form pink chromogen measured at 530-540 nm | Meat and meat products [52] | Sensitive; correlates with sensory rancidity | Lack of specificity; interference from other compounds [52] |
| Chromatographic Methods (GC, HPLC) | Volatile aldehydes, ketones, hydrocarbons | Separation and detection of volatile compounds | Meat, oils, various processed foods | High specificity and sensitivity | Expensive equipment; requires technical expertise [52] |
| p-Anisidine Value | Aldehydes (especially α,β-unsaturated) | Reaction with p-anisidine to form yellowish products | Oils and fats | Specific for aldehydes; complements PV | Less common; requires specific reagents |
Table 3: Indirect and Specialized Assessment Methods
| Method | Target Analytes | Principle | Typical Food Applications | Key Advantages | Key Limitations |
|---|---|---|---|---|---|
| Weight Gain | Oxygen uptake | Periodic measurement of mass increase during oxidation | Accelerated oxidation studies | Simple equipment; high capacity | Limited to controlled oxidation conditions [53] |
| Sensory Evaluation | Off-flavors, rancidity | Human perception of oxidation products | All food products | Direct relevance to consumer acceptance | Subjective; requires trained panelists [54] |
| Fluorometric Assay | Secondary oxidation products | Fluorescence measurement of oxidation products | Biological systems, some foods | High sensitivity | Indirect measure; interference possible [52] |
Experimental Protocols for Key Methodologies
Peroxide Value Determination via Iodometric Titration
Principle: This method quantifies hydroperoxides through their oxidation of iodide to iodine, which is then titrated with thiosulfate [52].
Reagents:
- Chloroform-methanol mixture (2:1 v/v)
- Saturated potassium iodide (KI) solution
- 0.01 N sodium thiosulfate (Na₂S₂O₃) solution
- 1% starch indicator solution
Procedure:
- Accurately weigh 2-5 g of oil sample into a 250 mL glass-stoppered conical flask.
- Add 20 mL of chloroform-methanol solution and swirl to dissolve the sample.
- Add 0.5 mL of saturated KI solution, stopper the flask, and swirl for 10 seconds.
- Let the solution stand in dark for exactly 10 minutes to allow complete reaction.
- Add 30 mL of distilled water and titrate immediately with 0.01 N Na₂S₂O₃ solution with vigorous shaking until the yellow color almost disappears.
- Add 0.5 mL of starch indicator and continue titration until the blue color disappears.
- Conduct a blank determination simultaneously.
Calculation: Peroxide value (meq O₂/kg) = [(S - B) × N × 1000] / W Where: S = sample titration volume (mL), B = blank titration volume (mL), N = normality of Na₂S₂O₃ solution, W = sample weight (g)
Critical Considerations:
- Perform under dim light to prevent further photo-oxidation
- Exclude oxygen during the reaction phase
- Use freshly prepared KI solution
- Complete titration rapidly after adding water [52]
TBARS (Thiobarbituric Acid Reactive Substances) Assay
Principle: Malondialdehyde and similar secondary oxidation products react with thiobarbituric acid to form a pink chromogen measurable at 530-540 nm [52].
Reagents:
- TBA solution: 0.02 M thiobarbituric acid in water
- Trichloroacetic acid (TCA) solution: 15% (w/v) in water
- Hydrochloric acid (HCl): 0.2 N solution
Procedure (Distillation Method):
- Homogenize 10 g food sample with 50 mL distilled water.
- Transfer to distillation flask with 47.5 mL additional water and 2.5 mL 4N HCl.
- Distill and collect 50 mL distillate.
- Mix 5 mL distillate with 5 mL TBA reagent in test tube.
- Heat mixture in boiling water bath for 35 minutes.
- Cool and measure absorbance at 530-540 nm against blank.
- Prepare standard curve using 1,1,3,3-tetraethoxypropane.
Procedure (Direct Extraction Method for Oils):
- Dissolve 100 mg oil in 25 mL isooctane.
- Add 5 mL TBA reagent (0.02 M in 90% acetic acid).
- Vortex mixture for 10 seconds.
- Heat at 95°C for 60 minutes.
- Cool and measure absorbance at 530-540 nm.
Calculation: TBARS value (mg MDA/kg) = (Asample - Ablank) × F / W Where: F = factor from standard curve, W = sample weight (kg)
Critical Considerations:
- Avoid iron contamination from homogenizer blades
- Control heating time and temperature precisely
- Account for interference from sugars, amino acids, and other TBA-reactive compounds [52]
Conjugated Diene Analysis
Principle: This method detects the formation of conjugated diene structures from polyunsaturated fatty acids during early oxidation stages by measuring UV absorption at 233-234 nm [52] [3].
Reagents:
- Isooctane or cyclohexane (UV grade)
- Methanol, HPLC grade
Procedure:
- Extract lipid from food sample using appropriate method (Folch for tissues, direct dissolution for oils).
- Accurately weigh 20-50 mg extracted lipid into 25 mL volumetric flask.
- Dissolve and dilute to volume with isooctane.
- Measure absorbance against isooctane blank at 233 nm.
- For samples with high absorbance, further dilute to ensure readings within linear range (0.1-1.0 AU).
Calculation: Conjugated diene value (μmol/g) = (A233 × V × 1000) / (ε × l × W) Where: A233 = absorbance at 233 nm, V = final volume (mL), ε = molar extinction coefficient (typically 25,200 L·mol⁻¹·cm⁻¹ for linoleic acid hydroperoxides), l = pathlength (cm), W = sample weight (mg)
Critical Considerations:
- Use UV-grade solvents to minimize background absorption
- Ensure complete lipid extraction for solid foods
- Account for inherent conjugated dienes in some unoxidized oils [3]
Method Selection Framework
Food Matrix-Specific Considerations
The composition and physical state of different food matrices significantly influence appropriate method selection:
Meat and Meat Products:
- TBARS method is widely applied due to correlation with warmed-over flavor development
- Peroxide value has limited utility for long-term storage due to hydroperoxide decomposition
- Chromatographic methods effective for detecting specific rancidity volatiles (hexanal, pentanal) [52]
Plant Oils:
- Peroxide value and conjugated diene methods effective for early oxidation monitoring
- p-Anisidine value useful for tracking aldehyde formation
- Weight gain method provides oxidative stability index under controlled conditions [53] [52]
Low-Fat Foods and Emulsions:
- Requires sensitive methods due to low oxidation product concentrations
- TBARS with concentration steps or solid-phase extraction
- Fluorometric methods for enhanced sensitivity [52]
Complex Processed Foods:
- Multiple methods often required due to ingredient complexity
- Sensory evaluation critical for consumer relevance
- Consider interference from non-lipid components in colorimetric assays [54]
Accuracy and Sensitivity Parameters
Method selection must consider the required sensitivity and accuracy for specific research objectives:
Table 4: Sensitivity and Detection Limits of Key Methods
| Method | Approximate Detection Limit | Linear Range | Precision (RSD%) | Key Interferences |
|---|---|---|---|---|
| Peroxide Value (Iodometric) | 0.1 meq/kg | 0.1-50 meq/kg | 3-8% | Oxygen, light, metal ions |
| Conjugated Dienes | 0.01 μmol/g | 0.01-10 μmol/g | 2-5% | Inherent conjugation in some oils |
| TBARS | 0.01 mg MDA/kg | 0.01-5 mg MDA/kg | 5-10% | Sugars, amino acids, pigments |
| GC-MS Volatiles | pg-level for specific compounds | Wide | 3-8% | Co-eluting compounds |
Integrated Assessment Approaches
Comprehensive lipid oxidation assessment typically requires multiple complementary methods:
- Primary Product Monitoring: PV and conjugated dienes for early oxidation
- Secondary Product Analysis: TBARS or specific carbonyl measurements for advanced oxidation
- Volatile Profiling: GC-MS for mechanistic studies and specific off-flavor compound identification
- Sensory Correlation: Human evaluation for consumer relevance
No single method comprehensively characterizes lipid oxidation status across all food matrices. Method selection should be guided by research objectives, matrix characteristics, and available resources.
The Scientist's Toolkit: Essential Research Reagent Solutions
Table 5: Key Reagents and Materials for Lipid Oxidation Analysis
| Reagent/Material | Function/Application | Critical Specifications | Storage/Stability Considerations |
|---|---|---|---|
| Thiobarbituric Acid (TBA) | TBARS assay for malondialdehyde detection | ≥98% purity; white crystalline powder | Store in dark at 4°C; prepare fresh solutions |
| 1,1,3,3-Tetraethoxypropane (TEP) | MDA standard for TBARS calibration | ≥96% purity; clear liquid | Store under inert gas; stable at 4°C |
| Sodium Thiosulfate | Titrant for peroxide value determination | Analytical grade; standardized solution | Store in dark, cool place; restandardize periodically |
| Chloroform-Methanol (2:1) | Lipid extraction medium (Folch method) | HPLC grade; stabilizer-free | Store in amber bottles; use in fume hood |
| Iron(II) Chloride | Catalyst in ferric thiocyanate method | Anhydrous, ≥99% purity | Desiccator storage; prepare fresh solutions |
| Potassium Iodide | Reductant in peroxide value assay | ≥99% purity; white crystals | Store in dark, cool place; prepare saturated solution fresh |
| n-Hexane/Isooctane | Solvent for UV analysis and lipid extraction | UV grade; low peroxide content | Store in amber bottles under inert atmosphere |
Workflow and Method Selection Pathways
Lipid Oxidation Pathways and Analytical Targets
Controlling Oxidation: Prooxidant Mitigation and Advanced Stabilization Strategies
Lipid oxidation is a dominant cause of rancidity in food systems, leading to the deterioration of sensory qualities, nutritional value, and shelf life of lipid-containing products [55]. This process initiates with the reaction of unsaturated fatty acids with reactive oxygen species (ROS), generating primary oxidation products like hydroperoxides and secondary products such as aldehydes, which are responsible for off-flavors and potential health risks [56] [55]. Antioxidants are crucial for mitigating this oxidative damage. They function primarily through two core mechanisms: free radical scavenging and metal chelation [57]. While the human body possesses endogenous antioxidant defenses, the prevailing view is that additional support from exogenous antioxidants—both natural and synthetic—is often necessary to maintain oxidative stability, particularly in complex systems like foods, feeds, and biological environments [58] [56]. This technical guide provides an in-depth analysis of the mechanisms, efficacy, and experimental evaluation of natural and synthetic antioxidants, framed within the context of lipid oxidation research.
Fundamental Mechanisms of Lipid Oxidation
Lipid oxidation is a chain reaction process that occurs in three main stages: initiation, propagation, and termination.
- Initiation: This stage begins when an initiating radical (X•) abstracts a hydrogen atom from a polyunsaturated fatty acid (LH), forming a lipid radical (L•). Key initiators include hydroxyl radicals (•OH) generated via Fenton reactions catalyzed by transition metals like Fe²⁺ or Cu²⁺ [56] [57].
- Propagation: The lipid radical (L•) rapidly reacts with molecular oxygen (O₂) to form a lipid peroxyl radical (LOO•). This highly reactive radical can then abstract a hydrogen atom from another lipid molecule (LH), generating a lipid hydroperoxide (LOOH) and a new lipid radical (L•), thereby propagating the chain reaction [56].
- Termination: The chain reaction concludes when two radicals combine to form a non-radical product. Antioxidants (AH) exert their effect primarily at this stage by donating a hydrogen atom to the peroxyl radical (LOO•), forming a more stable lipid hydroperoxide (LOOH) and a resonance-stabilized antioxidant radical (A•) that is insufficiently reactive to propagate the chain further [56] [57].
The following diagram illustrates this chain reaction and the pivotal role of antioxidants in its termination.
Core Antioxidant Mechanisms
Antioxidants counteract lipid oxidation through two primary, often complementary, mechanisms.
Free Radical Scavenging
This is the principal mechanism for primary antioxidants. These compounds donate a hydrogen atom (or an electron) to a free radical, such as a lipid peroxyl radical (LOO•), thereby neutralizing it and breaking the propagation chain of lipid oxidation [56] [57]. The effectiveness of a radical-scavenging antioxidant is largely determined by the bond dissociation energy (BDE) of the O-H bond in phenolic antioxidants; a lower BDE facilitates easier hydrogen donation [59]. The resulting antioxidant radical (A•) must be stable enough, often through resonance delocalization, to avoid acting as a pro-oxidant.
Metal Chelation
This is a key mechanism for secondary antioxidants. Transition metal ions like iron (Fe²⁺) and copper (Cu²⁺) are potent pro-oxidants that catalyze the initiation of lipid oxidation by decomposing hydroperoxides into reactive radicals [56] [57]. Metal chelators, such as citric acid, ethylenediaminetetraacetic acid (EDTA), and certain polyphenols, sequester these metal ions into stable complexes. This action inhibits the metal's redox activity, effectively suppressing both the initiation of new radical chains and the decomposition of existing hydroperoxides [57].
The interplay of these mechanisms within a composite antioxidant system can be visualized as follows:
Natural vs. Synthetic Antioxidants: A Comparative Analysis
The debate between natural and synthetic antioxidants is nuanced, with each category possessing distinct advantages and limitations. The following tables provide a detailed comparison of their properties and mechanisms.
Table 1: Comparative Analysis of Natural and Synthetic Antioxidants
| Viewpoint | Natural Antioxidants | Semi-Synthetic & Synthetic Antioxidants |
|---|---|---|
| Primary Mechanism | Radical scavenging (Phenolics, Vitamin E), Metal chelation (some polyphenols) [60] [56] | Radical scavenging (BHT), Metal chelation (EDTA), Radical stabilization (Ethoxyquin) [58] [57] |
| Efficiency in Foods | Variable; often low to moderate and highly matrix-dependent [58] [55] | Generally higher and more predictable in experimental and food models [58] [57] |
| Bioavailability | Often very low due to extensive metabolism and rapid elimination [58] | Generally better due to tailored design, though variable [58] |
| Toxicity & Safety | Generally low, but some can interfere with cytochrome P450 enzymes; high-dose supplements may have pro-oxidant effects [58] [56] | Some designed for lower toxicity, but cases of profound toxicity exist (e.g., troglitazone, high-dose EQ hepatotoxicity) [58] [57] |
| Key Advantages | Consumer preference ("clean-label"), multiple biological activities (e.g., anti-inflammatory) [60] [55] | Higher stability, tailored properties, cost-effectiveness, predictable performance [58] |
Table 2: Key Antioxidant Compounds and Their Properties
| Compound | Type | Primary Mechanism(s) | Key Features & Limitations |
|---|---|---|---|
| Vitamin E (e.g., α-Tocopherol) | Natural | Radical scavenging via H• donation to peroxyl radicals [58] | Essential nutrient; protects cell membranes; failed to show benefit in many intervention studies, with increased prostate cancer risk in some [58]. |
| Flavonoids/ Polyphenols | Natural | Radical scavenging, metal chelation (can act as pro-oxidants with metals) [60] [58] | Low bioavailability; beneficial effects may stem from non-antioxidant mechanisms (e.g., anti-inflammatory) [58]. |
| Butylated Hydroxytoluene (BHT) | Synthetic | Primary radical scavenger, terminating propagation chains via H• donation [57] | Effective but faces regulatory restrictions and consumer safety concerns; can volatilize during processing [55] [57]. |
| Ethoxyquin (EQ) | Synthetic | Stabilizes free radicals via intramolecular resonance delocalization [57] | Highly effective in suppressing propagation; associated with dose-dependent toxicity risks (e.g., hepatotoxicity) [57]. |
| Citric Acid | Natural / Synthetic | Metal chelation, sequestering pro-oxidant ions (Fe²⁺, Cu²⁺) [57] | Secondary antioxidant; provides continuous protection by suppressing initiation and propagation; often used synergistically [57]. |
Synergistic Effects in Composite Antioxidant Systems
A powerful strategy to overcome the limitations of single antioxidants is the use of rationally designed composite systems. These blends leverage synergism—where the combined effect is greater than the sum of individual effects [57]. A prominent example is the combination of a radical scavenger (e.g., BHT or EQ) with a metal chelator (e.g., citric acid).
- Mechanism of Synergism: The radical scavenger (primary antioxidant) neutralizes free radicals, while the metal chelator (secondary antioxidant) deactivates pro-oxidant metals. This dual action provides comprehensive, stage-specific protection throughout the oxidation process [57]. Furthermore, some components can help regenerate primary antioxidants.
- Experimental Evidence: A 2025 study demonstrated the superior performance of a ternary blend (Treatment E: 10 g/ton EQ + 12 g/ton BHT + 6 g/ton CA) compared to single antioxidants like BHT or EQ alone in stabilizing lipids in high-fat animal feed [57]. The composite system showed:
- Significantly enhanced retention of radical scavenging capacity.
- Most effective suppression of both primary oxidation (Peroxide Value) and secondary oxidation (Malondialdehyde, p-Anisidine Value).
- The lowest Total Oxidation (TOTOX) values under both natural storage and acute thermal stress.
- The ability to reduce the total antioxidant dosage while substantially extending the oxidative stability period, thereby mitigating toxicity risks associated with high doses of single components [57].
Experimental Evaluation and Protocols
Evaluating antioxidant efficacy and lipid oxidation status requires a multi-parametric approach, employing a suite of standardized assays.
Key Assays for Antioxidant Capacity and Oxidation Markers
Table 3: Key Research Reagent Solutions and Experimental Assays
| Assay / Reagent | Target / Function | Brief Protocol Overview & Application |
|---|---|---|
| DPPH Assay | Free radical scavenging capacity | Measures the decrease in absorbance at 517 nm as the purple DPPH• radical is reduced by an antioxidant [57]. |
| ABTS Assay | Radical absorbing capacity | Involves pre-generation of the blue-green ABTS•⁺ cation radical. Antioxidant capacity is determined by the extent of decolorization, measured at 734 nm [57]. |
| FRAP & CUPRAC | Reducing power | FRAP measures reduction of ferric (Fe³⁺) to ferrous (Fe²⁺) ions. CUPRAC uses cupric (Cu²⁺) to cuprous (Cu⁺) reduction. Both result in a colored complex measured spectrophotometrically [59]. |
| Peroxide Value (PV) | Primary oxidation products (Hydroperoxides) | Quantifies milliequivalents of peroxide oxygen per kilogram of fat, typically via titration with sodium thiosulfate after reaction with iodide [59] [57]. |
| p-Anisidine Value (p-AV) | Secondary oxidation products (Aldehydes) | Measures aldehydes (especially 2-alkenals) by their reaction with p-anisidine, resulting in a yellow color measured at 350 nm [57]. |
| Thiobarbituric Acid Reactive Substances (TBARS) | Secondary oxidation (Malondialdehyde, MDA) | MDA, a secondary oxidation product, reacts with TBA to form a pink chromogen measured at 532-535 nm. It is a common marker for lipid peroxidation [56] [57]. |
| Total Oxidation (TOTOX) Value | Overall oxidation status | A composite index calculated as TOTOX = 2PV + p-AV, providing a holistic assessment of both primary and secondary oxidation [57]. |
Advanced Analytical Techniques
Research relies on advanced analytical methods to gain deeper insights:
- Chromatography & Mass Spectrometry: Gas Chromatography-Mass Spectrometry (GC-MS) and Liquid Chromatography-Mass Spectrometry (LC-MS) are used for precise separation, identification, and quantification of specific oxidation products and volatile compounds [59] [55].
- Spectroscopy: Fourier-Transform Infrared (FTIR) spectroscopy monitors functional group changes (e.g., hydroperoxides), while Electron Spin Resonance (ESR) spectroscopy directly detects and identifies free radicals [59] [55].
The following workflow diagrams a typical experimental setup for evaluating antioxidant efficacy in a lipid system.
The choice between natural and synthetic antioxidants is not a simple binary decision. Synthetic antioxidants like BHT and EQ often provide potent, predictable, and cost-effective radical scavenging activity [58] [57]. However, concerns over safety and consumer preference are driving the industry towards natural alternatives [55]. Nevertheless, many natural antioxidants suffer from variable efficacy, low bioavailability, and complex matrices that can impede their performance [58]. The future of antioxidant strategies lies in leveraging a deep mechanistic understanding of lipid oxidation pathways. Rationally designed composite systems that harness the synergy between different mechanisms—such as combining radical scavengers with metal chelators—represent the most promising path forward [57]. This approach allows for reduced dosages of individual components, minimizes potential toxicity, and delivers comprehensive, stage-specific protection against lipid oxidation, ultimately enhancing the stability, safety, and shelf-life of a wide range of products.
The Role of Polyphenols and Plant Extracts as Clean-Label Alternatives
The global food industry is undergoing a significant transformation driven by consumer demand for natural, minimally processed ingredients. This shift towards "clean-label" products has positioned polyphenols and plant extracts as viable, sustainable alternatives to synthetic antioxidants in combating lipid oxidation and food rancidity. As secondary metabolites abundantly found in plants, polyphenols are recognized for their potent antioxidant and antimicrobial activities, which are crucial for extending the shelf-life of fat-rich foods while meeting consumer preferences for natural ingredients [61]. The move towards clean-label preservation is not merely a trend but a fundamental change in food production philosophy, emphasizing transparency, sustainability, and health-conscious formulations [62].
Within the context of lipid oxidation research, polyphenols offer a complex mechanism of action that goes beyond simple radical scavenging. Their multifaceted functionality includes chelating pro-oxidant metals, modulating oxidative enzymes, and interacting with food macromolecules to create protective barriers against rancidity development [63]. This technical review examines the mechanisms, sources, extraction methodologies, and applications of polyphenols as clean-label alternatives, with particular focus on their role in mitigating lipid oxidation in food systems.
Mechanisms of Action Against Lipid Oxidation
Primary Antioxidant Mechanisms
Polyphenols exert their primary antioxidant activity predominantly through free radical scavenging and electron transfer capabilities. Their chemical structure, characterized by aromatic rings and hydroxyl groups, enables the donation of hydrogen atoms to lipid radicals, thereby interrupting the propagation phase of lipid oxidation [63]. The antioxidant efficacy is directly influenced by the number and position of hydroxyl groups on the phenolic rings, which determines their redox properties and stability towards oxidation.
The fundamental reactions include:
- Radical Scavenging: R• + Ph-OH → RH + Ph-O•
- Peroxyl Radical Quenching: ROO• + Ph-OH → ROOH + Ph-O•
- Stabilization through Resonance: The phenoxyl radical formed after hydrogen donation is stabilized through electron delocalization across the conjugated system [64].
Secondary Antioxidant Mechanisms
Beyond direct free radical quenching, polyphenols exhibit secondary antioxidant functions that contribute significantly to their oxidative protective role:
Metal Chelation: Polyphenols, particularly those with ortho-dihydroxy groupings, can chelate transition metal ions such as Fe²⁺ and Cu⁺, which are potent catalysts for lipid hydroperoxide decomposition. This complex formation reduces the redox potential of these metals, thereby inhibiting the initiation of oxidative chains [63] [64].
Enzyme Inhibition: Certain polyphenol classes can inhibit pro-oxidant enzymes such as lipoxygenase, which catalyzes the oxidation of polyunsaturated fatty acids [63].
Synergistic Effects: Polyphenols can regenerate other antioxidants (e.g., vitamin E) through redox reactions, creating synergistic antioxidant systems that enhance overall oxidative stability [65].
Interactions with Food Matrices
The antioxidant efficiency of polyphenols is significantly influenced by their interactions with food components, particularly proteins. The formation of polyphenol-protein complexes can alter the structural and functional properties of both molecules. In myofibrillar protein gels, the incorporation of polyphenol-rich plant additives like blackcurrant pomace and Melissa officinalis extract resulted in enhanced antioxidant capacity while modifying textural properties [65]. These interactions can create protective barriers at oil-water interfaces or form antioxidant networks within the food matrix that collectively retard lipid oxidation progression.
Structural Classification of Polyphenols
Polyphenols encompass a diverse group of phytochemicals with over 200,000 identified structures, broadly categorized into:
- Flavonoids: Characterized by a C6-C3-C6 skeleton, including flavanols, flavonols, flavones, isoflavones, and anthocyanins [63] [64].
- Phenolic Acids: Derivatives of hydroxybenzoic and hydroxycinnamic acids.
- Stilbenes: Featuring a C6-C2-C6 structure (e.g., resveratrol).
- Lignans: Phenylpropanoid dimers.
- Tannins: High molecular weight compounds divided into hydrolysable and condensed tannins [64].
The structural diversity directly influences their antioxidant potential, bioavailability, and interaction with food components.
Polyphenols are widely distributed in plant kingdoms, with particularly high concentrations found in:
Table 1: Polyphenol-Rich Sources and Their Bioactive Components
| Source | Bioactive Polyphenols | Application in Food Systems |
|---|---|---|
| Oregano | Rosmarinic acid, chlorogenic acid, caffeic acid, quercetin, apigenin [63] | Meat preservation, edible films |
| Green Tea | Catechins, epigallocatechin gallate, procyanidins [66] | Oils, fat-rich products, active packaging |
| Grapes/Berries | Anthocyanins, proanthocyanidins, catechins, resveratrol [65] [66] | Meat products, functional food gels, beverages |
| Pomegranate Peel | Punicalagin, ellagic acid, anthocyanins [63] | Active packaging, meat preservation |
| Olive Mill Wastewater | Hydroxytyrosol, tyrosol, oleuropein [63] | Oil preservation, nutritional supplements |
| Sweet Potato Leaves | Caffeoylquinic acids, esculin, protocatechualdehyde [63] | Antioxidant extracts for various foods |
The valorization of agricultural by-products represents a sustainable approach for polyphenol extraction. Food processing wastes such as fruit peels, seeds, and other residual biomass contain substantial amounts of bioactive polyphenols, supporting a circular economy model in the food industry [63] [67].
Green Extraction Technologies
Conventional extraction methods using organic solvents are increasingly being replaced by green extraction technologies that reduce environmental impact while improving efficiency and selectivity [68].
Table 2: Green Extraction Techniques for Polyphenol Recovery
| Extraction Method | Principles | Optimal Conditions | Advantages |
|---|---|---|---|
| Microwave-Assisted Extraction (MAE) | Dielectric heating causing cell disruption [68] | 80-90°C, ~500 W, <3 min [68] | Reduced time, compatibility with green solvents |
| Ultrasound-Assisted Extraction (UAE) | Cavitation bubbles disrupting cell walls [68] | 40-45°C, <500 W, <30 min [68] | Lower energy consumption, higher yields |
| Pressurized Liquid Extraction (PLE) | High pressure and temperature maintaining solvent liquid state [68] | 50-200°C, 500-3000 psi [68] | Reduced solvent use, automation capability |
These advanced extraction methods have demonstrated significant improvements in polyphenol recovery yields. For instance, ultrasound-assisted extraction of spent coffee grounds increased polyphenol recovery by 33% while reducing energy consumption by half compared to conventional methods [68].
Applications in Food Preservation
Direct Incorporation in Food Matrices
The direct application of polyphenols in food products represents the most straightforward approach for exploiting their antioxidant potential. In meat and meat products, polyphenols from various plant extracts effectively inhibit lipid oxidation during processing, air-drying, and storage, addressing the susceptibility of these nutrient-rich matrices to rancidity [63]. The development of myofibrillar protein gels supplemented with polyphenol-rich plant additives demonstrates the potential for creating functional food systems with enhanced oxidative stability. Blackcurrant pomace significantly elevated both total polyphenol content and antioxidant capacity, while Melissa officinalis and Centella asiatica extracts further improved the nutritional profile and oxidative stability of the gels [65].
Active and Intelligent Packaging Systems
The incorporation of polyphenols into packaging materials represents an advanced application strategy that minimizes direct sensory impact while maintaining antioxidant efficacy at the food surface, where oxidation is most pronounced:
Active Packaging: Polyphenols embedded in packaging films or pads offer antibacterial, antioxidant, and antibrowning effects, extending shelf life while minimizing food wastage [67]. These systems can be designed for controlled release of bioactive compounds, maintaining effective concentrations throughout the product's shelf life.
Smart Packaging: Integrating polyphenols with indicators that monitor food condition provides real-time quality information. These systems utilize the pH-sensitive color-changing properties of anthocyanins to signal spoilage, creating communication channels between the product and consumer [67].
Intelligent Packaging: RFID tags and time-temperature indicators incorporating polyphenol-based sensors offer supply chain monitoring capabilities, addressing the significant challenge of temperature abuse during distribution and storage [67].
Regulatory and Market Landscape
The global polyphenol market, valued at USD 1203.84 million in 2024, is projected to reach USD 2576 million by 2033, growing at a compound annual growth rate (CAGR) of 8.82% [66]. This growth is largely driven by the surge in demand for natural additives and clean-label products, with the functional foods segment owning the highest market share [66]. Simultaneously, the natural food antioxidants market is expected to grow at a CAGR of 6.4% from 2025 to 2033, reaching USD 2.1 billion [69].
Regulatory approvals have been instrumental in facilitating this growth. The U.S. Food and Drug Administration's classification of polyphenols as Generally Recognized as Safe (GRAS), along with similar approvals from the European Food Safety Authority, has encouraged innovation and application development [66]. The emergence of the "purple food revolution" capitalizing on anthocyanin-rich sources further exemplifies the market traction of polyphenol-based solutions [66].
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents and Materials for Polyphenol Research
| Research Reagent/Material | Function/Application | Technical Notes |
|---|---|---|
| ABTS/Trolox Reagents | Quantification of antioxidant capacity via radical cation decolorization assay [65] | Standard for comparing antioxidant potential across extracts |
| DPPH Radical Solution | Assessment of free radical scavenging activity [65] | Simple, rapid screening method for antioxidant activity |
| FRAP Reagents | Measurement of ferric reducing antioxidant power [65] | Evaluates electron-transfer capability of polyphenols |
| Folin-Ciocalteu Reagent | Determination of total phenolic content [65] | Spectrophotometric quantification relative to gallic acid equivalents |
| Myofibrillar Protein Isolate | Model system for studying protein-polyphenol interactions [65] | Critical for understanding macromolecular interactions in food matrices |
| Edible Film Forming Solutions | Development of active packaging (e.g., chitosan, gelatin, PLA-based) [67] | Vehicle for controlled release of polyphenols in packaging applications |
| Nanocarrier Systems | Nanoencapsulation to improve stability and bioavailability (nanoliposomes, solid lipid nanoparticles) [63] | Enhances functionality and targeted delivery of polyphenols |
Polyphenols and plant extracts represent a scientifically sound and commercially viable solution for addressing the persistent challenge of lipid oxidation in food systems while meeting the growing consumer demand for clean-label products. Their multifaceted mechanisms of action, diverse natural sources, and compatibility with advanced extraction and application technologies position them as indispensable tools in modern food preservation. The integration of polyphenols into direct food applications and innovative packaging systems offers a sustainable path forward for extending shelf life, reducing food waste, and enhancing food quality and safety. Future research directions should focus on optimizing extraction efficiencies, elucidating structure-activity relationships in complex food matrices, and developing targeted delivery systems that maximize antioxidant potential while minimizing sensory impacts. As the scientific understanding of polyphenol mechanisms advances and technologies for their application mature, these natural compounds are poised to play an increasingly central role in the global transition toward cleaner, safer, and more sustainable food preservation strategies.
Lipid oxidation is a paramount cause of quality deterioration in food and pharmaceutical products, leading to rancidity, nutrient degradation, and formation of potentially harmful compounds. This chemical process, initiated when unsaturated lipids react with molecular oxygen, compromises sensory properties, nutritional value, and safety profiles. Within the broader context of food rancidity research, physical barrier strategies represent critical technological interventions that directly target the fundamental requirement for oxidation: oxygen availability. Modified Atmosphere Packaging (MAP) and oxygen scavengers constitute two sophisticated approaches that engineer the packaging environment to prevent or delay oxidative reactions. These technologies are particularly vital for products rich in polyunsaturated fatty acids, including nutraceuticals, lipid-based drug delivery systems, and functional foods, where oxidative stability directly correlates with product efficacy and shelf life. This technical guide examines the scientific principles, implementation methodologies, and research applications of these physical barrier strategies, providing researchers with a comprehensive framework for their evaluation and implementation.
Technological Fundamentals and Anti-Oxidation Mechanisms
Modified Atmosphere Packaging (MAP)
MAP functions by altering the gaseous composition surrounding a product to create an environment hostile to oxidative degradation and microbial proliferation. Unlike ambient packaging, which contains approximately 21% oxygen, 78% nitrogen, and 0.03% carbon dioxide, MAP employs customized gas mixtures tailored to specific product requirements [70]. The primary gases utilized include carbon dioxide (CO₂) for its bacteriostatic and fungistatic properties, nitrogen (N₂) as an inert filler gas to prevent package collapse, and in specific cases, low levels of oxygen (O₂) for products requiring respiratory metabolism or oxymyoglobin preservation [71] [72].
The anti-oxidation mechanism of MAP is fundamentally rooted in oxygen exclusion or reduction. By lowering the partial pressure of oxygen in the headspace, MAP reduces the driving force for oxygen permeation into the product and decreases the dissolved oxygen available for lipid oxidation reactions. Carbon dioxide provides additional protection by increasing the solubility of lipids in bacterial cell membranes, thereby disrupting cellular function and extending the lag phase of spoilage microorganisms [72]. The efficacy of a specific MAP configuration is quantifiable through the oxygen transmission rate (OTR) of the packaging material, typically reported in cm³/m²/day, and the actual dissolved oxygen content in the product matrix, which can be monitored using non-destructive oxygen sensors.
Oxygen Scavengers
Oxygen scavengers represent an active packaging technology that chemically removes oxygen from the package headspace and product matrix through irreversible reactions. These systems provide a complementary or alternative approach to MAP, particularly effective against residual oxygen that remains after packaging or permeates through packaging materials during storage [73]. Unlike the passive barrier provided by MAP materials, oxygen scavengers actively consume oxygen molecules, creating a dynamic protection system that adapts to changing conditions.
The mechanisms employed by leading oxygen scavenger technologies include:
- Iron-Based Oxidation: The most prevalent commercial systems utilize powdered iron oxide (FeO) which oxidizes to ferric oxide (Fe₂O₃) in the presence of moisture, typically achieving oxygen absorption capacities of 20-300 mL O₂ [73].
- Enzymatic Scavenging: Systems incorporating glucose oxidase or alcohol oxidase catalyze the oxidation of specific substrates while consuming molecular oxygen. These offer food-compatible profiles but have narrower activation parameters [73].
- Light-Activated Scavengers: Photosensitizers like titanium dioxide (TiO₂) become activated under UV light, generating reactive oxygen species that subsequently react with scavenger substrates. These allow for controlled activation timing but require light exposure [73].
- Polymer-Integrated Systems: Ascorbate-based or other organic compound scavengers are incorporated directly into polymer matrices, enabling package structures with inherent oxygen scavenging capabilities without separate sachets [73].
The kinetics of oxygen scavenging follow pseudo-first-order reaction models initially, transitioning to zero-order kinetics as the scavenging capacity is depleted. The performance is quantified by scavenging capacity (mL O₂/g scavenger) and scavenging rate (mL O₂/g/hour), both highly dependent on temperature, relative humidity, and the specific product matrix.
Table 1: Comparative Analysis of Oxygen Barrier Technologies
| Technology Parameter | Modified Atmosphere Packaging | Oxygen Scavengers |
|---|---|---|
| Primary Mechanism | Displacement of oxygen with inert gases | Chemical or enzymatic binding of oxygen molecules |
| Initial O₂ Reduction | Rapid (during packaging process) | Gradual (hours to days post-packaging) |
| Residual O₂ Management | Limited to initial reduction | Continuously addresses residual and permeating O₂ |
| Typical Oxygen Levels Achieved | 0.2-5.0% in headspace | <0.01% in headspace |
| Activation Requirements | Gas flushing equipment | Moisture, UV light, or pH triggers (technology-dependent) |
| Shelf Life Extension Potential | 1.5-4x conventional packaging | 2-5x conventional packaging |
| Technology Integration | Primary packaging systems | Sachets, labels, polymer-integrated, bottle crowns |
Quantitative Performance Data and Research Findings
Efficacy Metrics in Food Model Systems
Recent research provides quantitative evidence of the protective effects of MAP and oxygen scavengers against lipid oxidation. In a 2024 study investigating meat analogue products stored in MAP (35% CO₂, 65% N₂), thiobarbituric acid reactive substances (TBARS) values varied significantly among product types on the final day of shelf life, demonstrating the interaction between formulation and packaging efficacy. Minced analogue products showed superior oxidative stability with TBARS values of 3.20 mg MDA·kg⁻¹, while filet-type analogues were more susceptible to oxidation, displaying TBARS values of 9.71 mg MDA·kg⁻¹ [71]. This underscores the importance of product-specific packaging optimization.
Research on fish models demonstrates even more pronounced benefits. A 2025 study on Atlantic bonito (Sarda sarda) fillets compared MAP (80% N₂ + 20% CO₂) against vacuum packaging, sodium alginate coating, and refrigerated controls. After 15 days of storage at 4°C, MAP-treated samples exhibited significantly lower TBARS values (1.29 ± 0.06 mg MDA/kg) and peroxide values (1.10 ± 0.05 mEq/kg) compared to other treatments, confirming the enhanced oxidative stability provided by modified atmospheres specifically engineered for high-fat marine oils [72].
High Barrier Packaging Material Performance
The efficacy of both MAP and oxygen scavenger technologies is contingent upon the barrier properties of the primary packaging material. High barrier films are engineered to provide superior protection against external oxygen, moisture vapor, and light. The market for these advanced materials is projected to grow from USD 1.3 billion in 2025 to USD 2.2 billion by 2035, reflecting increased adoption across food and pharmaceutical sectors [74].
Table 2: Oxygen Barrier Film Materials and Performance Characteristics
| Material Type | Oxygen Transmission Rate (OTR) | Moisture Vapor Transmission Rate (MVTR) | Key Applications | Sustainability Profile |
|---|---|---|---|---|
| Metalized Films | <0.1 cc/m²/day at 23°C, 0% RH | <0.1 g/m²/day at 37.8°C, 90% RH | Snacks, crisps, coffee, pharmaceuticals | Difficult to recycle; some compostable innovations available |
| EVOH Copolymers | 0.1-0.2 cc/m²/day at 23°C, 0% RH | Highly dependent on relative humidity | Liquid nutraceuticals, lipid supplements | Recyclable in multilayer structures; bio-based variants in development |
| PVDC Coatings | 0.5-1.0 cc/m²/day at 23°C, 0% RH | 0.5-1.5 g/m²/day at 37.8°C, 90% RH | Meat, cheese, pharmaceutical blister packs | Recycling challenges; regulatory scrutiny in some regions |
| Transparent High Barrier Films | 0.5-5.0 cc/m²/day at 23°C, 0% RH | 1.0-5.0 g/m²/day at 37.8°C, 90% RH | Bakery goods, confectionery, fresh pasta | Mono-material options enhance recyclability |
| Compostable Barrier Films | 1.0-10.0 cc/m²/day at 23°C, 0% RH | 5.0-20.0 g/m²/day at 37.8°C, 90% RH | Short-shelf-life organic products, fresh snacks | Home/industrial compostable; TIPA 312 MET, T.LAM series [75] |
The oxygen transmission rate (OTR) represents the primary metric for evaluating barrier performance, with high-barrier films defined as those with OTR values below 1 cc/m²/day under standard conditions (23°C, 0% relative humidity) [75] [76]. Metallized films lead the market with approximately 35% share due to their superior barrier properties and cost efficiency, while transparent films are experiencing accelerated growth driven by consumer preference for product visibility and improved recycling characteristics [76].
Research Methodologies and Experimental Protocols
Standardized Testing Protocols for Lipid Oxidation Assessment
Research evaluating the efficacy of physical barrier strategies requires standardized methodologies to quantify oxidative progression and product stability. The following protocols represent established approaches cited in current literature:
Thiobarbituric Acid Reactive Substances (TBARS) Assay This widely-employed method quantifies malondialdehyde (MDA), a secondary lipid oxidation product. The standardized protocol involves:
- Homogenizing 10 g sample with 95.7 mL distilled water and 2.5 mL 4N HCl for 2 minutes [71].
- Distilling the homogenate and collecting approximately 50 mL distillate.
- Mixing 5 mL distillate with 5 mL TCA reagent (15% trichloroacetic acid, 0.375% thiobarbituric acid).
- Incubating in boiling water bath for 35 minutes followed by cooling.
- Measuring absorbance at 532 nm against appropriate blank.
- Calculating TBARS value (mg MDA·kg⁻¹ sample) by multiplying absorbance by factor 7.8 [71].
Peroxide Value (PV) Determination This method measures hydroperoxides, primary products of lipid oxidation:
- Dissolving an appropriate sample weight (1-5 g depending on expected oxidation level) in chloroform:acetic acid (2:3) mixture.
- Adding saturated potassium iodide solution and incubating in darkness for exactly 5 minutes.
- Adding 30 mL distilled water and titrating with standardized 0.01N sodium thiosulfate using starch indicator.
- Calculating peroxide value as mEq O₂/kg sample based on titration volume [72].
Headspace Gas Composition Analysis Critical for MAP studies, this protocol verifies and monitors package atmosphere:
- Utilizing a calibrated gas analyzer (e.g., Check Point II, PBI Dansensor) with appropriate sample needle [71].
- Inserting probe through specialized septum or dedicated sampling port.
- Recording multiple measurements per package to ensure representative sampling.
- Reporting mean values for O₂, CO₂, and N₂ percentages with standard deviations.
Experimental Design Considerations
Robust experimental design for evaluating physical barrier strategies should incorporate:
- Appropriate controls (air-packed samples, vacuum packaging)
- Multiple sampling intervals throughout projected shelf life
- Replication accounting for biological and technical variability
- Realistic storage conditions (temperature, humidity, light exposure)
- Complementary microbial analysis where applicable
- Sensory evaluation correlated with chemical measurements
Diagram 1: Mechanism of lipid oxidation pathways and packaging intervention points. Physical barrier strategies target specific stages of the oxidative cascade to preserve product quality.
Advanced Research Applications and Innovation Frontiers
Integrated Packaging Systems and Synergistic Effects
Current research explores the synergistic potential of combining multiple physical barrier technologies. Integrated MAP systems incorporating oxygen scavengers demonstrate enhanced efficacy by addressing both initial oxygen reduction and continuous scavenging of permeating oxygen. A 2025 study on bison meat demonstrated that CO₂/N₂ modified atmosphere master bag packaging systems with integrated oxygen scavengers significantly enhanced shelf life while maintaining color stability [77]. This combination approach is particularly valuable for high-value products requiring extended shelf life or those with exceptional oxygen sensitivity.
Research indicates that the sequential activation of barrier technologies can optimize protection throughout the product lifecycle. Light-activated oxygen scavengers that initiate only after retail display begins, combined with initial MAP flushing, provide temporal coordination of protection phases. Similarly, humidity-activated systems in dried products become functional only after potential moisture ingress, preserving scavenging capacity until needed.
Sustainable Material Innovations
The sustainability imperative is driving innovation in barrier packaging technologies, with research focusing on renewable, compostable, and recyclable alternatives that maintain performance standards:
- Compostable High-Barrier Films: Advanced materials like TIPA's compostable polymers and coated cellulose-based films provide viable alternatives to conventional plastics while maintaining functional barrier properties. These materials align with circular economy principles through certified home and industrial compostability [75].
- Bio-Based Coatings: Research at Nofima investigates coatings derived from plant starches, alginate from seaweed, and chitosan from shellfish for cellulose-based packaging. While promising, these materials face challenges in maintaining barrier integrity when contacting high-moisture or high-fat foods [78].
- Mono-Material Structures: The development of mono-material packaging with inherent barrier properties addresses recycling challenges posed by traditional multilayer structures. Polyethylene and polypropylene-based systems with enhanced barrier coatings maintain recyclability while providing sufficient protection for many applications [74].
Diagram 2: Comprehensive experimental workflow for evaluating packaging efficacy. Integrated assessment methodologies provide multidimensional data for optimization decisions.
The Researcher's Toolkit: Essential Materials and Methods
Table 3: Research Reagent Solutions for Oxidation and Packaging Studies
| Reagent/Category | Specific Examples | Research Application | Technical Considerations |
|---|---|---|---|
| Oxidation Indicators | Thiobarbituric acid, Potassium iodide, Ferric chloride | Quantification of primary and secondary lipid oxidation products | TBARS requires acidic conditions and heating; PV measurements must avoid light exposure |
| Gas Mixtures | Food-grade CO₂, N₂, O₂, specialized blends (e.g., 35% CO₂/65% N₂) | MAP system optimization and control atmosphere creation | Precision gas mixing equipment required; certification for purity essential |
| Oxygen Scavengers | Iron-based sachets, UV-activated films, ascorbate-integrated polymers | Active packaging systems comparison and synergy studies | Activation requirements vary; capacity testing prerequisite |
| Barrier Materials | Metallized PET, EVOH multilayers, PVDC-coated films, compostable alternatives | Permeation studies and material selection optimization | Pre-conditioning at test RH critical; seal integrity affects results |
| Analytical Instruments | Gas chromatographs, Oxygen headspace analyzers, Texture analyzers, Spectrophotometers | Objective quantification of package atmosphere and product quality | Regular calibration with standards essential; validation protocols required |
Physical barrier strategies comprising Modified Atmosphere Packaging and oxygen scavenging technologies represent scientifically validated approaches to mitigate lipid oxidation in oxygen-sensitive products. The efficacy of these systems is quantifiable through standardized chemical assays monitoring oxidative progression, with recent research demonstrating significant shelf-life extensions across diverse product categories. Successful implementation requires product-specific optimization accounting for composition, storage conditions, and distribution requirements. Future innovation trajectories include intelligent scavenging systems with triggered activation, sustainable barrier materials maintaining performance profiles, and integrated approaches leveraging synergistic protection mechanisms. For researchers investigating food rancidity mechanisms, these technologies provide potent interventions for controlling oxidative variables while maintaining product integrity throughout designated shelf life.
The preservation of food and pharmaceutical products represents a critical challenge for researchers and industry professionals. Within this context, the chemical degradation of lipids through oxidation stands as a primary mechanism compromising product quality, safety, and shelf life. Lipid oxidation is a complex process wherein unsaturated fatty acids undergo deterioration when exposed to environmental factors, leading to rancidity and the formation of potentially harmful compounds [4] [18]. This technical guide examines the fundamental pathways of lipid oxidation and establishes evidence-based protocols for optimizing storage parameters to mitigate these deleterious reactions. The control of temperature, light, and moisture—three catalytic factors in lipid degradation—forms the cornerstone of effective preservation strategies across the supply chain [79] [80]. By framing these parameters within the mechanistic context of lipid oxidation, this whitepaper provides a scientific foundation for developing robust storage protocols that maintain product integrity from production to consumption.
Mechanisms of Lipid Oxidation: A Scientific Foundation
Lipid oxidation proceeds through several well-characterized pathways, each with distinct initiators and reaction products. Understanding these mechanisms is essential for developing targeted storage interventions.
Autoxidation: The Free Radical Chain Reaction
Autoxidation constitutes the primary pathway for lipid deterioration in stored products. This self-sustaining chain reaction comprises three distinct phases:
- Initiation: The reaction initiates when external factors (heat, light, metal ions) cause the removal of a hydrogen atom from unsaturated fatty acids, forming lipid radicals (L•). Notably, pre-existing lipid hydroperoxides (LOOH) can decompose to provide the initial radicals, especially in the presence of metal catalysts [4] [80].
- Propagation: Lipid radicals (L•) rapidly react with molecular oxygen (³O₂) to form peroxyl radicals (LOO•). These radicals then abstract hydrogen from adjacent fatty acids, generating new lipid radicals and lipid hydroperoxides (LOOH), thereby propagating the chain reaction [4]. The propagation rate increases significantly once hydroperoxide concentration reaches a critical micelle concentration, enabling transfer between lipid colloids [80].
- Termination: The reaction chain terminates when radicals combine to form non-radical products (e.g., L• + L• → L-L) [4].
Table 1: Primary Lipid Oxidation Pathways and Their Characteristics
| Pathway | Initiators | Primary Products | Secondary Products |
|---|---|---|---|
| Autoxidation | Heat, light, metal ions | Lipid hydroperoxides | Aldehydes, ketones, hydrocarbons |
| Photo-oxidation | Light (especially UV), photosensitizers | Lipid hydroperoxides | Similar to autoxidation but different isomer distribution |
| Hydrolytic | Moisture, lipase enzymes | Free fatty acids, glycerol | Short-chain fatty acids (e.g., butyric acid) |
| Enzymatic | Lipoxygenase enzymes | Hydroperoxides | Aldehydes, dicarboxylic acids |
| Microbial | Microorganisms, lipases | Free fatty acids | Ketones, aldehydes |
Secondary Oxidation and Ramifications
Hydroperoxides formed during primary oxidation are unstable and decompose into numerous secondary products, including aldehydes, ketones, alcohols, and hydrocarbons [4]. These compounds are responsible for the characteristic off-flavors and odors associated with rancidity. Notably, certain aldehydes such as malondialdehyde (MDA) serve as biomarkers for oxidative stress and can potentially form adducts with proteins, raising concerns about nutritional quality and safety [4]. The decomposition products depend on temperature, oxygen concentration, and the specific fatty acid profile of the lipid [4].
Storage Parameter Optimization
Strategic management of storage conditions directly targets the initiation and propagation mechanisms of lipid oxidation. The following evidence-based recommendations provide a framework for optimizing these critical parameters.
Temperature Control
Temperature profoundly influences the rate of lipid oxidation, with both absolute temperature and fluctuation contributing to degradation.
Table 2: Temperature Optimization Guidelines for Lipid Stability
| Product Category | Recommended Range | Critical Considerations | Monitoring Protocol |
|---|---|---|---|
| Frozen goods | -18°C to -25°C | Minimize temperature fluctuations during storage transitions | Continuous monitoring with alerts for deviations >2°C |
| Refrigerated products | 0°C to 4°C | Maintain stability despite door openings and stock rotation | Multi-point mapping to identify stratification zones |
| Room-temperature stable | <21°C | Protect from thermal peaks near heating systems | Strategic sensor placement away from direct sunlight |
| Thermosensitive pharmaceuticals | Product-specific (e.g., 2-8°C) | Validate stability through accelerated aging studies | Real-time monitoring with data logging for compliance |
Refrigerated warehouses demonstrate the practical application of these principles, where strategic placement of stock-keeping units (SKUs) according to their thermal requirements minimizes quality degradation. Products with strict thermal requirements should be positioned in zones with stable cooling, while less sensitive items may be stored in peripheral areas [79]. Advanced monitoring employing Internet of Things (IoT) sensors enables real-time temperature mapping to identify stratification zones where warmer air accumulates vertically [79]. This spatial approach to temperature management represents a significant advancement over uniform temperature setpoints.
Light Management
Light, particularly in the ultraviolet and visible spectrum, initiates photo-oxidation through two primary mechanisms: direct absorption by double bonds in unsaturated lipids, and photosensitization where light-absorbing compounds (e.g., chlorophyll, riboflavin) transfer energy to molecular oxygen, generating singlet oxygen (¹O₂) [4] [18]. This highly reactive species directly attacks double bonds, forming hydroperoxides at rates up to 1500 times faster than autoxidation [4].
Protective strategies include:
- Packaging opacity: Amber or opaque packaging provides superior protection compared to clear materials. Aluminum foil packaging offers complete light exclusion.
- Spectral filtering: Incorporating UV filters in transparent packaging materials blocks high-energy wavelengths while maintaining product visibility.
- Storage environment: Implementing dark storage conditions or using light-emitting diodes (LEDs) with minimal UV emission in display areas.
Humidity and Moisture Control
The role of moisture in lipid oxidation is complex, exhibiting both pro-oxidative and protective effects depending on the food system and concentration. Humidity control targets three degradation pathways:
- Hydrolytic rancidity: In this moisture-dependent process, ester linkages in triglycerides undergo cleavage, releasing free fatty acids [18]. The reaction is catalyzed by lipases, acids, or bases, and accelerates with increasing water activity [18].
- Microbial activity: High water activity (a_w > 0.6) supports microbial growth, where microorganisms secrete lipases that catalyze fat breakdown [18].
- Antioxidant functionality: The "polar paradox" describes how antioxidant effectiveness depends on their solubility relative to the oxidation site [80]. Polar antioxidants are more effective in bulk oils, while nonpolar antioxidants work better in emulsified systems. Humidity affects this delicate balance by altering the physicochemical environment at lipid interfaces.
Optimization models for refrigerated warehouses now integrate both temperature and humidity constraints, recognizing their interconnected impact on product quality [79]. Recommended relative humidity levels typically range between 60-70% for most dry goods, though specific thresholds should be established based on a product's critical water activity.
Assessment Methodologies and Experimental Protocols
Rigorous assessment of lipid oxidation is essential for validating storage protocols and establishing product shelf life. The following methodologies represent standard approaches in research and quality control.
Primary Oxidation Product Analysis
Peroxide Value (PV) Determination
- Principle: Measures hydroperoxides, the primary products of lipid oxidation [4].
- Protocol (Iodometric titration):
- Dissolve 5 g lipid sample in 30 mL acetic acid-chloroform solution (3:2 v/v).
- Add 0.5 mL saturated potassium iodide solution.
- Flush with nitrogen, seal, and incubate in darkness for 5 minutes with occasional shaking.
- Add 30 mL distilled water and titrate with 0.01 N sodium thiosulfate using starch indicator.
- Include blank determination without sample.
- Calculate PV = (S - B) × N × 1000 / sample weight (g), where S and B are sample and blank titration volumes, and N is sodium thiosulfate normality.
- Applications: Suitable for meat, edible insects, and oil-based products during early and intermediate oxidation stages [4].
Conjugated Diene Analysis
- Principle: Detects conjugation of double bonds in polyunsaturated fatty acids that occurs during hydroperoxide formation [4].
- Protocol:
- Dilute lipid sample in cyclohexane or isooctane to achieve absorbance <1.2.
- Measure absorbance at 233 nm against solvent blank.
- Calculate conjugated diene value = A × dilution factor / (c × l), where A is absorbance, c is sample concentration (g/L), and l is path length (cm).
- Applications: Particularly useful for polyunsaturated fatty acid-containing foods during early oxidation [4].
Secondary Oxidation Product Analysis
Thiobarbituric Acid Reactive Substances (TBARS) Assay
- Principle: Quantifies malondialdehyde (MDA) and other secondary oxidation products that react with thiobarbituric acid [4].
- Protocol:
- Homogenize 10 g sample with 50 mL distilled water.
- Transfer aliquot to distillation apparatus with 47.5 mL distilled water and 2.5 mL 4N HCl.
- Distill until 50 mL collected.
- Mix 5 mL distillate with 5 mL 0.02M TBA solution.
- Incubate in boiling water bath for 35 minutes.
- Cool and measure absorbance at 532-535 nm.
- Quantify using standard curve prepared with 1,1,3,3-tetraethoxypropane.
- Applications: Widely used for meat, fish, and edible insect products [4].
p-Anisidine Value Test
- Principle: Measures secondary aldehydic compounds, particularly α,β-unsaturated aldehydes [4].
- Protocol:
- Dissolve 0.5-4.0 g oil in 25 mL iso-octane (depending on expected value).
- Measure initial absorbance at 350 nm (A₁).
- Mix 5 mL solution with 1 mL 0.25% p-anisidine in glacial acetic acid.
- After 10 minutes, measure absorbance at 350 nm (A₂).
- Calculate p-Anisidine Value = 25 × (1.2A₂ - A₁) / sample weight (g).
- Applications: Often used in conjunction with PV to calculate total oxidation (TOTOX) value [4].
Accelerated Stability Testing
Rancimat Method
- Principle: Accelerates oxidation by heating sample with continuous air bubbling, measuring conductivity change in effluent water [18].
- Protocol:
- Weigh 3 g sample into reaction tube.
- Heat to predetermined temperature (typically 90-120°C).
- Pass purified air (20 L/h) through sample into measuring vessel containing distilled water.
- Continuously monitor conductivity of water.
- Record induction time as point of rapid conductivity increase.
- Applications: Standardized method for fats and oils (AOCS Cd 12b-92, ISO 6886) [18].
Visualizing Lipid Oxidation Pathways and Storage Impacts
Oxidation Pathways and Storage Impacts
Advanced Considerations in Storage Optimization
Interfacial Phenomena in Lipid Oxidation
The "polar paradox" theory elucidates the complex behavior of antioxidants in different lipid systems, with critical implications for storage stability [80]. Polar antioxidants demonstrate superior efficacy in bulk oils by accumulating at air-oil interfaces where oxidation initiates, while nonpolar antioxidants prove more effective in oil-in-water emulsions by protecting lipid droplets from aqueous phase pro-oxidants [80]. This principle extends to the "cut-off effect," where antioxidant effectiveness increases with alkyl chain length until a critical point, beyond which efficacy declines due to reduced mobility, internalization into lipid cores, or self-aggregation [80]. Storage protocols must consider these interfacial phenomena, particularly for emulsified products or those containing endogenous or added antioxidants.
Emerging Technologies and Oxidation Risks
Innovative food processing technologies present paradoxical oxidation risks despite their environmental and efficiency benefits [80]:
- Ohmic heating: While superior for nutrient retention, electrochemical reactions at electrode surfaces may generate free radicals that initiate lipid oxidation [80].
- High-pressure processing: Alters cell membrane integrity and releases compartmentalized pro-oxidants, potentially accelerating oxidation in lipid-rich tissues [80].
- Ultrasound and microwave processing: Cavitation and thermal effects can generate localized hotspots and free radicals despite reduced overall processing times [80].
These technologies necessitate tailored storage protocols that account for their unique degradation pathways, often requiring modified atmosphere packaging or enhanced antioxidant strategies to mitigate induced oxidation.
The Researcher's Toolkit: Essential Reagents and Materials
Table 3: Key Research Reagents and Materials for Lipid Oxidation Studies
| Reagent/Material | Function | Application Notes |
|---|---|---|
| Thiobarbituric acid | Reacts with malondialdehyde to form colored complex | Prepare fresh solution; light-sensitive |
| p-Anisidine reagent | Reacts with α,β-unsaturated aldehydes | Use high-purity reagent; prepare in glacial acetic acid |
| Potassium iodide | Reduces hydroperoxides in PV determination | Prepare saturated solution; store in amber bottles |
| Sodium thiosulfate | Titrant for iodine in PV method | Standardize frequently; protect from light and air |
| Cyclohexane/Isooctane | Solvents for spectrophotometric analysis | Use UV-spectrophotometric grade |
| Antioxidants (BHT/BHA) | Prevents further oxidation during analysis | Add to solvents during extraction |
| Lipoxygenase enzyme | Enzyme-catalyzed oxidation studies | Use specific activity; store at -20°C |
| Fatty acid standards | Reference materials for chromatography | Prepare stock solutions under nitrogen |
The optimization of storage parameters for lipid-containing products demands a mechanistic understanding of oxidation pathways coupled with rigorous monitoring and intervention strategies. Temperature control remains the cornerstone of preservation, with strategic management minimizing both primary oxidation initiation and secondary decomposition reactions. Light exclusion strategies directly target photo-oxidation pathways, while humidity control addresses hydrolytic rancidity and microbial activity. Advanced assessment methodologies enable precise quantification of oxidation states, facilitating evidence-based storage protocol development. As emerging processing technologies introduce novel oxidation risks, and supply chains grow increasingly complex, the integration of these fundamental principles with real-time monitoring systems represents the future of quality preservation for researchers and industry professionals committed to delivering safe, high-quality products.
The preservation of food and pharmaceutical products against oxidative deterioration represents a critical challenge for industry and researchers alike. This whitepaper examines the advanced strategies combining complementary antioxidant systems with innovative processing technologies to significantly enhance oxidative stability. Through detailed mechanistic insights and experimental evidence, we demonstrate how rationally designed antioxidant blends—leveraging regeneration cycles, complementary partitioning, and dual mechanisms—coupled with novel activation technologies can overcome the limitations of single-component systems. The integration of these approaches provides a robust framework for extending product shelf-life, maintaining nutritional quality, and ensuring safety across diverse applications from functional foods to pharmaceutical formulations.
Lipid oxidation remains a formidable challenge for the food and pharmaceutical industries despite more than 150 years of research, primarily due to the complexity of products and multiple elements influencing oxidation pathways [81]. This process generates volatile compounds including aldehydes, ketones, and alcohols that cause rancidity, while simultaneously promoting co-oxidation of proteins, vitamins, and pigments, thereby compromising nutritional quality, texture, and appearance [81]. Beyond quality deterioration, lipid oxidation products demonstrate potential toxicity to biological tissues, raising significant health concerns [81].
The susceptibility of polyunsaturated fatty acids (PUFAs) to oxidative degradation presents particular challenges for products rich in these nutritionally valuable components. For instance, pearl millet flour, despite its nutritional superiority as a "nutricereal" containing 5–5.4% fats with 70–80% unsaturated fatty acids, suffers from rapid degradation through both hydrolytic and oxidative rancidity, severely limiting its shelf-life and commercial potential [9]. Similarly, fish oil, valued for its high omega-3 PUFA content, is exceptionally vulnerable to oxidative deterioration due to the multiple double bonds in eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), with oxidation rates increasing exponentially with each additional double bond [7].
Biological tissues naturally possess highly efficient endogenous antioxidant systems protecting aerobic organisms against oxygen and its reactive species. However, conventional food processing operations often remove or degrade these protective systems through mechanisms such as thermal destruction of antioxidant enzymes, steam stripping of phenolic compounds, and refining processes that remove beneficial antioxidants from oils [81]. This fundamental understanding of both oxidation mechanisms and natural protection systems provides the foundation for developing advanced stabilization strategies combining exogenous antioxidants with processing technologies designed to enhance stability.
Mechanisms of Antioxidant Synergism
Fundamental Synergistic Interactions
Antioxidant combinations produce three possible interactions: synergism, where the combined effect exceeds the sum of individual effects; additive effect, where no additional benefit or harm occurs; and antagonism, where the combination proves less effective than the sum of individual components [81]. True synergistic interactions provide significant advantages for product formulation, including extended shelf-life, reduced required antioxidant concentrations (lowering costs and potential toxicity), and enhanced nutritional quality and safety [81].
The most impactful synergistic combinations typically involve antioxidants with complementary mechanisms and physical properties. These systems often pair:
- Primary free radical scavengers with metal chelators that inhibit initiation of oxidation
- Hydrophobic antioxidants positioned at oxidation sites with hydrophilic antioxidants capable of regenerating them
- Fast-reacting antioxidants with slow-reacting antioxidants that provide sustained protection
The regeneration of primary antioxidants through redox cycling represents one of the most well-established synergistic mechanisms. In these systems, a primary antioxidant (e.g., α-tocopherol) scavenges free radicals and becomes oxidized, while a secondary antioxidant (e.g., ascorbic acid) regenerates the primary antioxidant back to its active reduced form [81]. This regeneration cycle significantly extends the antioxidant capacity beyond what either component could achieve independently. The "pecking order" of free radicals and antioxidants follows fundamental thermodynamic principles where antioxidants with lower reduction potentials can reduce those with higher potentials, creating efficient electron transfer cascades [81].
Spatial Partitioning and Matrix Effects
The physical location of antioxidants within complex food systems profoundly influences their effectiveness, giving rise to synergistic interactions based on differential partitioning behavior. Antioxidants distribute themselves throughout the food matrix according to their polarity, with non-polar antioxidants concentrating in lipid phases and polar antioxidants accumulating in aqueous regions [81]. This natural partitioning can be strategically exploited by combining antioxidants with different polarity profiles to provide comprehensive protection throughout heterogeneous food systems.
Research demonstrates that the synergistic effects between phospholipids and tocopherols originate from this spatial partitioning, where phospholipids position themselves at oil-water interfaces—primary sites of oxidation initiation—and subsequently regenerate tocopherols through hydrogen donation [81]. Similarly, the synergistic interaction between albumin and green tea catechins in oil-in-water emulsions emerges from the protein's ability to locate catechins at the interface where oxidation predominantly occurs [81].
The food matrix itself significantly influences synergistic outcomes, with factors including pH, interfacial characteristics, antioxidant concentration ratios, and interactions with other food components collectively determining the ultimate effectiveness of antioxidant combinations [81]. This complexity explains why predicting synergistic combinations remains challenging and underscores the necessity for mechanistic understanding rather than empirical screening approaches.
Advanced Processing Technologies
Field-Based Activation Technologies
Emerging technologies utilizing combined field induction show significant promise for enhancing antioxidant activity and stability in food products. Research on organic carrot juices has demonstrated that mixed-field technologies (incorporating plasmatic, magnetic, and gravitational fields) can induce a high natural energy charge suitable for extended shelf-life [82]. These technologies appear to activate valuable bio-compounds with antioxidant value, which oxidize first, thereby protecting the product environment [82].
This activation approach optimizes reaction mechanisms and synergistic effects during processing, resulting in final products with enhanced compositional stability during long-term storage [82]. Furthermore, the application of these technologies has demonstrated unexpected benefits, including the development of protective mechanisms against microorganisms such as Escherichia coli in final juice products [82]. The underlying mechanism may involve driving redox processes controlled by NADH+H+- or FMNH+H+-dependent oxidoreductases to areas of low redox potential, creating both antimicrobial and anti-helminthic effects [82].
Extraction and Stabilization Techniques
Supercritical fluid extraction (SFE) has emerged as a valuable technology for obtaining antioxidant compounds while preserving their activity. This method is particularly advantageous for thermolabile compounds that might be degraded by conventional extraction processes [82]. When combined with activated bio-membranes, SFE enables optimized extraction operations and activation of valuable biocomponents [82].
The integration of advanced analytical technologies provides unprecedented insights into oxidation processes and antioxidant efficacy. Lipidomics, defined as the comprehensive analysis of lipid species within biological systems, utilizes advanced techniques such as mass spectrometry coupled with chromatographic separation to enable high-throughput identification and quantification of numerous lipid molecules [7]. Similarly, flavoromics systematically profiles and analyzes volatile organic compounds that contribute to aroma characteristics, utilizing gas chromatography–mass spectrometry and olfactometry to identify trace-level volatile compounds associated with oxidative deterioration [7].
Experimental Approaches and Methodologies
Assessment of Oxidative Stability
Comprehensive evaluation of lipid oxidation requires a multi-parametric approach incorporating analysis of primary oxidation products, secondary products, and integrated indices that provide holistic assessment [83]. The following experimental protocols represent standardized methodologies for assessing oxidative stability in complex matrices.
Table 1: Standard Analytical Methods for Lipid Oxidation Assessment
| Parameter | Method | Principle | Application Context |
|---|---|---|---|
| Peroxide Value (PV) | Titration (GB 5009.227-2016) | Measures hydroperoxides (primary oxidation products) | Early-stage oxidation monitoring [7] |
| p-Anisidine Value (p-AV) | Spectrophotometry (GB/T 24304-2009) | Measures unsaturated aldehydes (secondary oxidation) | Secondary oxidation assessment [83] [7] |
| Conjugated Dienes (CD) | Spectrophotometry (AOCS Ti 1a-64) | Detects formation of conjugated dienes from PUFAs | Early detection of oxidation [83] |
| Acid Value (AV) | Titration (GB 5009.229-2016) | Measures free fatty acids from hydrolytic rancidity | Hydrolytic degradation [7] |
| Malondialdehyde (MDA) | TBARS assay (GB 5009.181-2016) | Quantifies MDA as secondary lipid oxidation product | Advanced oxidation monitoring [83] [7] |
| Total Oxidation (TOTOX) | Calculated: 2 × PV + p-AV | Integrates primary and secondary oxidation | Overall oxidation status [83] [7] |
Accelerated Stability Testing Protocols
Accelerated oxidation tests provide valuable predictive data for product shelf-life without requiring extended storage periods. The Schaal oven test represents one widely employed approach, where samples are maintained at 60°C and monitored periodically for oxidation markers [7]. This method accelerates the oxidation process while maintaining relevance to real-world degradation pathways.
For more severe stress conditions, thermal treatment at 120°C for 2 hours followed by ambient storage provides rapid assessment of antioxidant efficacy under extreme conditions [83]. This approach is particularly valuable for evaluating performance in applications involving high-temperature processing or cooking.
Experimental workflows for comprehensive stability assessment integrate multiple analytical approaches to provide complete understanding of antioxidant performance and oxidative deterioration pathways.
Antioxidant Efficacy Assessment
Standardized methods for evaluating antioxidant activity provide crucial data for comparing different formulations and understanding structure-activity relationships. The most widely employed assays include:
- DPPH Radical Scavenging Assay: Measures hydrogen donation capacity to stable DPPH radical [84] [83]
- ABTS Radical Scavenging Assay: Determines ability to quench pre-formed ABTS radical cation [84] [83]
- Ferric Reducing Antioxidant Power (FRAP): Assesses reduction potential toward ferric ions [84]
- Total Antioxidant Capacity: Comprehensive evaluation using multiple radical species [83]
These assays should be conducted following standardized protocols using commercial assay kits to ensure reproducibility and comparability across studies [83]. When evaluating synergistic effects, comparison of experimental values to theoretical additive values identifies true synergism (experimental > theoretical), additive effects (experimental = theoretical), or antagonism (experimental < theoretical).
Quantitative Evidence of Synergistic Efficacy
Ternary Antioxidant Systems in Feed Matrices
A comprehensive study evaluating synergistic efficacy of composite antioxidants in high-fat animal feed demonstrated superior performance of rationally formulated ternary blends compared to single-component systems [83]. The experimental design incorporated both natural storage conditions (25°C for 10 weeks) and acute thermal stress (120°C for 2 hours followed by ambient storage) to evaluate performance under diverse conditions.
Table 2: Synergistic Efficacy of Antioxidant Blends in Feed Matrices Under Thermal Stress
| Treatment | Composition | DPPH Retention (%) | PV Reduction (%) | MDA Reduction (%) | TOTOX Value |
|---|---|---|---|---|---|
| Control | No antioxidant | 100 (baseline) | 0 (baseline) | 0 (baseline) | 185.6 |
| A | 36 g/ton BHT | 132.5 | 28.4 | 31.2 | 142.3 |
| B | 60 g/ton EQ | 141.7 | 32.6 | 29.8 | 138.5 |
| C | 132 g/ton EQ | 138.2 | 25.7 | 24.3 | 145.1 |
| E | 10 g/ton EQ + 12 g/ton BHT + 6 g/ton CA | 165.3 | 45.2 | 52.7 | 102.4 |
The ternary blend (Treatment E) demonstrated significantly enhanced retention of radical scavenging capacity, with DPPH retention 19-25% higher than single antioxidants after 10 weeks of storage [83]. More notably, this combination most effectively suppressed both primary oxidation (peroxide value) and secondary oxidation (malondialdehyde), achieving 45% and 53% reductions respectively compared to control [83]. This comprehensive protection resulted in the lowest total oxidation (TOTOX) values across all conditions, confirming the temperature-resilient protection delivered by the synergistic system combining radical quenching and metal chelation mechanisms [83].
Efficacy in Fish Oil Stabilization
Lipidomics and flavoromics approaches have provided molecular-level insights into antioxidant efficacy in highly unsaturated systems such as fish oil. Research on silver carp viscera oil demonstrated that tertiary butylhydroquinone (TBHQ) and propyl gallate (PG) at 0.02% concentration significantly attenuated the oxidative degradation of triacylglycerols (TG), diacylglycerols (DG), and phosphatidylethanolamines (PE) containing unsaturated fatty acids [7].
This protection at the molecular level translated to practical quality preservation, with these antioxidants markedly suppressing the formation of six characteristic off-flavor compounds: 1-octen-3-ol, N-nonyl aldehyde, (E, E)-2,4-heptadienal, (E)-2-nonenal, (E)-2-decenal, and eugenol, thereby preventing the development of fishy odor [7]. Multivariate statistical analysis established PE, TG, and DG molecular species as critical precursors in the antioxidant-mediated suppression of volatile off-flavor generation, providing novel mechanistic insights into the dual protective role of antioxidants in maintaining both lipid integrity and flavor quality [7].
The Scientist's Toolkit: Essential Research Reagents and Methodologies
Table 3: Key Research Reagent Solutions for Antioxidant Synergism Studies
| Category | Specific Reagents | Function & Application | Experimental Notes |
|---|---|---|---|
| Synthetic Antioxidants | Butylated hydroxytoluene (BHT), Ethoxyquin (EQ), tert-butylhydroquinone (TBHQ) | Radical scavenging reference compounds; baseline protection | Dose-dependent toxicity concerns with EQ; BHT volatility limitations [83] |
| Natural Antioxidants | Tea polyphenols (TP), propyl gallate (PG), tocopherols | Clean-label alternatives; diverse mechanism of action | TP contains ~65% epigallocatechin-3-gallate as primary active [7] |
| Metal Chelators | Citric acid (CA), phosphates, EDTA | Pro-oxidant inactivation; prevention of initiation | Enhances primary antioxidants; enables reduced dosage [83] |
| Oxidation Indicators | DPPH, ABTS, FRAP reagents | Free radical scavenging capacity assessment | Standardized commercial kits ensure reproducibility [83] |
| Chromatography Standards | Supelco 37 FAME mix, lipid internal standards | Lipid profiling; quantification reference | Essential for lipidomics approaches [7] [85] |
| Accelerated Testing Materials | Schaal oven setup, precision temperature controls | Predictive shelf-life testing | 60°C standard for oils; 120°C for thermal stress [83] [7] |
The strategic combination of antioxidants with complementary mechanisms and targeted processing technologies represents a paradigm shift in oxidative stability management. The evidence presented demonstrates that rationally designed composite systems—particularly those combining radical scavengers with metal chelators—consistently outperform single-component antioxidants across diverse applications from animal feed to fish oil preservation.
Future research directions should focus on several key areas: First, the development of more sophisticated analytical approaches, particularly integrated lipidomics-flavoromics platforms, will provide deeper mechanistic understanding of antioxidant function in complex matrices. Second, the optimization of physical field technologies for antioxidant activation warrants expanded investigation to establish standardized protocols and elucidate underlying molecular mechanisms. Finally, the continued identification and characterization of novel natural antioxidant sources, coupled with advanced delivery systems to enhance bioavailability and targeted action, will support industry trends toward clean-label formulations while maintaining efficacy.
The convergence of these approaches—mechanistically designed antioxidant systems, advanced processing technologies, and sophisticated analytical methodologies—provides a powerful framework for addressing the persistent challenge of oxidative deterioration across food and pharmaceutical applications. By transcending traditional empirical approaches in favor of rationally designed stabilization strategies, researchers and product developers can significantly enhance product quality, safety, and commercial viability.
Biomarkers and Health Impacts: Validating Oxidation in Nutritional and Biomedical Contexts
Lipid oxidation is a fundamental chemical process that poses a significant challenge to food quality, affecting sensory attributes, nutritional value, and shelf-life of lipid-containing products [3]. This complex process involves the reaction of unsaturated fatty acids with reactive oxygen species, generating both primary and secondary oxidation products that contribute to rancidity and potential health risks [55]. Among the numerous compounds formed during lipid oxidation, three biomarkers have emerged as critical indicators of oxidative status: malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), and oxysterols. These biomarkers not only serve as indicators of oxidative damage in food systems but also possess biological significance, with implications for human health and disease pathogenesis [86] [87]. Understanding their formation pathways, analytical detection methods, and occurrence in foods is essential for researchers, scientists, and drug development professionals working to mitigate oxidative rancidity and its associated effects.
The assessment of lipid oxidation biomarkers provides valuable insights into the mechanisms of food rancidity, enabling the development of effective stabilization strategies and antioxidant systems [55]. Recent advances in analytical techniques, including chromatography, spectroscopy, and volatile compound profiling, have significantly enhanced our ability to detect and quantify these biomarkers across various food matrices with improved precision and sensitivity [88] [55]. This technical guide comprehensively examines the current knowledge on MDA, 4-HNE, and oxysterols, with emphasis on their formation pathways, analytical methodologies, occurrence in food systems, and toxicological significance.
Malondialdehyde (MDA)
Formation Pathways and Significance
Malondialdehyde (MDA) is one of the most abundant and well-studied secondary products of lipid peroxidation, commonly used to indicate the degree of lipid oxidation in foods [89]. MDA exists in food systems in two main forms: free malondialdehyde (FrMDA) and protein-bound malondialdehyde (PrMDA), which exists in unstable Schiff base conjugates [89]. In meat matrices, the protein-bound form predominates, with linkages between MDA and amino acids that can be hydrolyzed by either acid or alkali treatments [89]. The quantification of MDA in food-related studies typically measures the total amount of both forms.
MDA is generated through the peroxidation of polyunsaturated fatty acids (PUFAs) containing three or more double bonds [3]. The mechanism involves the formation of lipid hydroperoxides as primary oxidation products, which subsequently degrade through carbon-carbon cleavage adjacent to the hydroperoxide group, yielding MDA among other carbonyl compounds [3]. This degradation process is influenced by factors such as temperature, oxygen concentration, and the presence of pro-oxidants or antioxidants in the food matrix.
Analytical Methods for MDA Detection
The analysis of MDA in food products has evolved significantly, with various methodological approaches developed to improve accuracy and sensitivity. The table below summarizes the primary analytical techniques used for MDA detection in food matrices:
Table 1: Analytical Methods for Malondialdehyde (MDA) Detection in Foods
| Method | Principle | Detection Limit | Advantages | Limitations |
|---|---|---|---|---|
| Spectrophotometric TBA Assay | Reaction with thiobarbituric acid (TBA) to form pink chromogen measured at 532 nm [89] | Varies with matrix | Simple, cost-effective, high-throughput | Lacks specificity; interference from other TBARs [89] |
| HPLC with Fluorescence Detection | Separation of MDA-TBA adduct by reversed-phase chromatography [90] | Picomole levels [90] | Improved specificity over spectrophotometric method | Requires derivatization; matrix effects |
| GC-MS with PFPH Derivatization | Derivatization with perfluorophenylhydrazine followed by GC-MS separation [89] | 0.25 ng/mL [89] | High sensitivity and specificity; uses deuterated internal standard | Complex sample preparation; expensive instrumentation |
| SFC-ESI-QqQ-MS/MS with DNPH Derivatization | Derivatization with DNPH followed by supercritical fluid chromatography separation [88] | Not specified | Simultaneous analysis of multiple aldehydes; minimal solvent consumption | Method relatively new; requires validation |
The traditional thiobarbituric acid (TBA) assay remains one of the most commonly used methods for determining MDA content, despite its limitations [89]. This method involves adding a high concentration of trichloroacetic acid (TCA) to hydrolyze PrMDA, followed by reaction with TBA at approximately 90°C under acidic conditions to form a pink TBA-MDA complex measurable at 532 nm [89]. However, TBA lacks specificity for MDA and reacts with various other compounds classified as thiobarbituric acid reactive substances (TBARs), including phenolics, water-soluble proteins and peptides, other carbonyl compounds from oxidized lipids, sucrose, and pigments [89]. This lack of specificity often leads to overestimation of MDA content.
Chromatographic techniques have significantly improved the precision of MDA detection. Improved GC-MS methods utilizing deuterium-substituted MDA (MDA-d₂) as an internal standard and perfluorophenylhydrazine (PFPH) as a derivative reagent have demonstrated particular effectiveness in complex matrices, with recovery rates between 93.9% and 98.4% and coefficients of variation for intermediate precision between 1.1% and 3.5% [89]. This method effectively reduces deviations from the presence of nitrite in cured meat products like salted lean pork meat, where nitrite can react with MDA under acidic conditions, leading to underestimation [89].
More recently, supercritical fluid chromatography coupled with triple quadrupole mass spectrometry (SFC-QqQ-MS/MS) has been developed for simultaneous determination of MDA and other α,β-unsaturated aldehydes after derivatization with 2,4-dinitrophenylhydrazine (DNPH) [88]. This method offers advantages including low solvent consumption and excellent limits of detection and quantification, accuracy, and precision [88].
Experimental Protocol: GC-MS Analysis of MDA with PFPH Derivatization
The following detailed protocol describes an improved GC-MS method for accurate MDA quantification in complex food matrices, particularly those containing nitrite:
Standard Solution Preparation: Prepare MDA and MDA-d₂ standard solutions as hydrolyzed products from 1,1,3,3-tetraethoxypropane (TEP) and TEP-d₂, respectively. Determine concentrations by absorbance at 244 nm (ε = 13,700) [89].
Sample Hydrolysis:
- For acid hydrolysis: Use trichloroacetic acid (TCA) to hydrolyze protein-bound MDA.
- For alkaline hydrolysis: Use sodium hydroxide solution. Alkaline hydrolysis may be preferable for samples containing nitrite to minimize interference [89].
Derivatization: React the hydrolyzed sample with perfluorophenylhydrazine (PFPH) to form MDA-PFPH derivatives. Optimize reaction time and temperature for maximum yield.
Extraction: Extract derivatives with an appropriate organic solvent (e.g., n-hexane).
GC-MS Analysis:
- Injection: 1 μL in splitless mode
- Column: Equity-5 or equivalent (30 m × 0.25 mm i.d., 0.25 μm film thickness)
- Temperature program: Hold at 50°C for 1 min, increase to 180°C at 15°C/min, then to 280°C at 5°C/min, final hold for 5 min.
- Ion source temperature: 230°C
- Interface temperature: 280°C
- Selected ion monitoring (SIM): m/z 234 for MDA and m/z 236 for MDA-d₂ [89].
Quantification: Use the internal standard method with calibration curves prepared from MDA standards.
Occurrence in Foods and Toxicological Significance
MDA is found in varying concentrations in numerous food products, particularly in meat and meat products, fried foods, and edible oils. The European Food Safety Authority (EFSA) has established a toxicity threshold of 30.0 μg/kg of body weight per day for MDA [88]. In pickled and cured meat products, the accuracy of MDA quantification can be compromised by the presence of nitrite, which reacts with MDA under acidic conditions [89]. This reaction leads to underestimation of MDA content and consequently an underestimation of lipid oxidation in these products.
4-Hydroxy-2-nonenal (4-HNE)
Formation Pathways and Chemical Properties
4-Hydroxy-2-nonenal (4-HNE) is a highly reactive α,β-unsaturated aldehyde considered one of the most toxic products of lipid peroxidation [86]. It is classified under 4-hydroxy-2-alkenals due to the substitution of a hydroxyl group at the C4 position [86]. The compound contains three main functional groups: a carbonyl group, a C=C double bond, and a hydroxyl group, all of which can participate in chemical reactions with biological molecules [86]. The conjugated system of a C=C double bond and a C=O carbonyl group provides a partial positive charge to C3, making it susceptible to nucleophilic attack [86].
4-HNE is primarily derived from the decomposition of ω-6 polyunsaturated fatty acids, including linoleic acid and arachidonic acid, via both enzymatic and non-enzymatic mechanisms [86]. The non-enzymatic formation occurs through oxygen radical-dependent reactions involving lipid hydroperoxides (LOOH), alkoxyl radicals, epoxides, and fatty acyl crosslinking reactions [86]. During lipid peroxidation, lipid hydroperoxides are rapidly produced through free radical chain reaction mechanisms. Protonation of LOOH leads to the formation of alkoxyl radicals, which subsequently undergo β-scission to produce 4-HNE along with other aldehydes [86].
Analytical Methods for 4-HNE Detection
The analysis of 4-HNE in food matrices presents challenges due to its high reactivity and low concentration. The table below summarizes the primary analytical approaches for 4-HNE detection:
Table 2: Analytical Methods for 4-Hydroxy-2-nonenal (4-HNE) Detection in Foods
| Method | Principle | Key Features | Applications |
|---|---|---|---|
| HPLC-UV | Direct detection at 220-224 nm [86] | Simple; no derivatization required | Limited sensitivity; matrix interference |
| GC-MS | Derivatization followed by separation and mass detection [86] | High sensitivity and specificity | Requires extensive sample preparation |
| LC-MS | Direct separation and tandem mass spectrometry detection [86] | High sensitivity; minimal sample preparation | Expensive instrumentation; matrix effects |
| SFC-ESI-QqQ-MS/MS with DNPH | Derivatization with DNPH followed by SFC separation [88] | Simultaneous analysis of multiple aldehydes | Emerging technique; limited validation |
Classical methods for 4-HNE analysis include spectrophotometric methods and gas or liquid chromatography coupled to mass spectrometry [86]. 4-HNE shows a specific wavelength absorption maximum at 220-224 nm, enabling detection by HPLC with a UV detector [86]. Prior to injection, the analyte is typically extracted from food with water and cleaned with octadecyl silica gel columns [86].
More advanced techniques involve LC-MS/MS approaches that provide enhanced sensitivity and specificity. Additionally, the recently developed SFC-ESI-QqQ-MS/MS method with DNPH derivatization enables simultaneous determination of 4-HNE alongside other α,β-unsaturated aldehydes including MDA [88]. This method has been applied successfully to various oily foods and edible oils, providing critical insights into the formation patterns of these toxic aldehydes during thermal processing [88].
Experimental Protocol: LC-MS/MS Analysis of 4-HNE
The following protocol describes a comprehensive method for 4-HNE quantification in food matrices using liquid chromatography-tandem mass spectrometry:
Sample Extraction:
- Homogenize food sample (1 g) with 5 mL of aqueous solution containing 0.5% butylated hydroxytoluene (BHT) to prevent artificial oxidation during analysis.
- Centrifuge at 10,000 × g for 15 minutes at 4°C.
- Collect supernatant for solid-phase extraction.
Solid-Phase Extraction Cleanup:
- Condition C18 SPE cartridge with methanol followed by water.
- Load sample extract onto cartridge.
- Wash with water and elute 4-HNE with methanol.
- Evaporate eluent under gentle nitrogen stream and reconstitute in mobile phase.
LC-MS/MS Analysis:
- Column: C18 reversed-phase column (150 × 2.1 mm, 2.6 μm)
- Mobile phase: (A) 0.1% formic acid in water, (B) 0.1% formic acid in acetonitrile
- Gradient: 20% B to 95% B over 15 minutes, hold 5 minutes
- Flow rate: 0.3 mL/min
- Injection volume: 10 μL
- Ionization: ESI negative mode
- Multiple reaction monitoring (MRM): m/z 155.1 → 137.0 (quantifier), 155.1 → 109.0 (qualifier) [86] [88].
Quantification: Use standard addition method or external calibration with matrix-matched standards.
Occurrence in Foods and Toxicological Significance
4-HNE occurs in various food products, with the highest concentrations typically found in vegetable oils, fried potatoes, and meat products [86]. The European Food Safety Authority has established a threshold of toxicological concern level for 4-HNE exposure at 1.5 μg/kg body weight/day [86] [88]. This aldehyde has been associated with several disease pathologies due to its high reactivity with biological macromolecules. 4-HNE can readily form Michael adducts with nucleophilic functional groups in proteins, DNA, and phospholipids, leading to cellular dysfunction [86] [91]. The compound has been implicated in various diseases including adult respiratory distress syndrome, atherogenesis, diabetes, and cancer [86].
Oxysterols
Formation Pathways and Classification
Oxysterols are oxidation products of cholesterol and phytosterols formed through various oxidative mechanisms in food systems. Sterols can oxidize in the B ring due to the presence of a double bond at position 5,6 or in the side-chain due to the presence of a double bond or tertiary carbons [87]. Sterol oxidation in food occurs through three primary reaction mechanisms: autoxidation, photosensitized oxidation, and enzymatic oxidation [87].
Autoxidation initiates with the abstraction of a reactive allylic hydrogen at C7, giving rise to a radical molecule that reacts with triplet oxygen (³O₂) to form a 7-peroxy radical [87]. This radical neutralizes with a hydrogen radical from other sterol/fatty acid molecules, generating 7α-/7β-hydroperoxysterols. After dysmutation of 7-hydroperoxysterols, 7α-/7β-hydroxysterols and 7-ketosterol are formed [87]. Other major sterol autoxidation products derive from the bimolecular reaction between a hydroperoxy radical and an unoxidized sterol molecule, leading to the formation of 5α,6α-/5β,6β-epoxysterols, which can undergo an oxirane ring opening in presence of water in acidic conditions and convert into sterol triols [87].
Photosensitized oxidation is much faster (>1500 times) and starts with the ene-addition of singlet oxygen (¹O₂) on either side of the double bond in the B ring, generating 5α-/6α-/6β-hydroperoxysterols, of which 5α-OOH is the most abundant and rearranges to the more stable 7α-OOH isomer [87]. Recent research has focused on photosensitized oxidation of sterols during LED exposure, as fluorescent lamps are being replaced in all food retailers [87].
Major Oxysterols and Their Significance
The most common oxysterols found in food products include 7-ketocholesterol, 7α- and 7β-hydroxycholesterol, 5,6α- and 5,6β-epoxycholesterol, and cholestanetriol [87]. Among all cholesterol oxidation products (COPs), 7-ketocholesterol (7-KC) is frequently used as a marker of cholesterol oxidation in foods due to its stability and relatively high concentration in oxidized cholesterol-containing products [87].
Table 3: Major Oxysterols in Food Products and Their Significance
| Oxysterol | Formation Pathway | Significance | Common Food Sources |
|---|---|---|---|
| 7-Ketocholesterol | Autoxidation of cholesterol [87] | Marker of cholesterol oxidation; cytotoxic effects | Heated dairy, eggs, meat products |
| 7α-/7β-Hydroxycholesterol | Autoxidation and photosensitized oxidation [87] | Primary oxidation products; pro-inflammatory effects | Dairy products, eggs, meat |
| 5,6α-/5,6β-Epoxycholesterol | Bimolecular reaction [87] | Precursors to cholestanetriol; cytotoxic | Processed foods with cholesterol |
| Cholestanetriol | Hydrolysis of epoxycholesterol [87] | Highly cytotoxic; associated with atherosclerosis | Aged cholesterol-rich foods |
Analytical Methods for Oxysterol Analysis
The analysis of oxysterols typically involves extraction, purification, and chromatographic separation followed by detection. The following protocol describes a comprehensive method for oxysterol analysis:
Sample Extraction:
- Add internal standards (e.g., deuterated oxysterols) to homogenized food sample (0.5 g).
- Saponify with ethanolic KOH solution (1 M) at 60°C for 45 minutes to release bound oxysterols and remove triglycerides.
- Extract unsaponifiable matter with hexane/diethyl ether mixture.
Solid-Phase Extraction Cleanup:
- Use silica gel SPE cartridges for normal-phase separation.
- Elute with hexane/diethyl ether mixtures of increasing polarity.
- Collect oxysterol fraction for derivatization.
Derivatization:
- Convert oxysterols to trimethylsilyl (TMS) ether derivatives using BSTFA or MSTFA.
- Heat at 60°C for 30 minutes.
- Evaporate under nitrogen and reconstitute in appropriate solvent for GC analysis.
GC-MS Analysis:
- Column: Non-polar capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness)
- Temperature program: 150°C to 300°C at 3-5°C/min
- Ionization: EI mode at 70 eV
- Selected ion monitoring (SIM) for specific oxysterol fragments [87].
Alternatively, LC-MS methods can be employed for direct analysis without derivatization, offering the advantage of analyzing thermally labile oxysterols that may degrade during GC analysis.
Occurrence in Foods and Health Implications
Oxysterols are found in various cholesterol-containing foods, with dairy, eggs, meat, and fish products being the primary sources [87]. When these foods undergo processing, particularly thermal processing, they become significant dietary sources of cholesterol oxidation products (COPs). The extent of COP generation is mainly related to processing temperatures and times, storage conditions, handling, and packaging [87]. Furthermore, COPs are unintentionally formed in household preparations, becoming a potential dietary risk for humans.
The biological effects of oxysterols have gained increasing attention due to their associations with human disease development, particularly cardiovascular diseases, cancer, neurodegenerative disorders, and autoimmune diseases [87]. The role of COPs as simple, quick, and non-invasive biomarkers in diagnosis has been proposed, with higher plasma levels observed in Parkinson's, coronary artery disease, and atherosclerosis patients [87]. The action mechanisms of COPs depend on their chemical and biophysiological nature as well as the cell-line or tissue tested [87].
Comparative Analysis and Research Applications
Method Selection Guide for Different Research Applications
Selecting the appropriate analytical method for lipid oxidation biomarkers depends on the specific research objectives, matrix complexity, and available instrumentation. The table below provides guidance on method selection for different research scenarios:
Table 4: Method Selection Guide for Lipid Oxidation Biomarker Analysis
| Research Application | Recommended Methods | Key Considerations |
|---|---|---|
| Routine Quality Control | Spectrophotometric TBA assay for MDA; PV and AnV for general oxidation | Throughput, cost-effectiveness, simplicity |
| Mechanistic Studies | GC-MS with derivatization; LC-MS/MS for multiple biomarkers | Specificity, sensitivity, comprehensive profiling |
| Toxicological Assessment | SFC-ESI-QqQ-MS/MS for multiple aldehydes; GC-MS for oxysterols | Accurate quantification, low detection limits, regulatory compliance |
| Clinical/Nutritional Studies | HPLC with fluorescence/UV detection; LC-MS/MS | Biomarker validation in biological samples, precision |
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful analysis of lipid oxidation biomarkers requires specific reagents and materials optimized for each analytical approach:
Table 5: Essential Research Reagents and Materials for Lipid Oxidation Biomarker Analysis
| Reagent/Material | Application | Function | Technical Notes |
|---|---|---|---|
| Thiobarbituric Acid (TBA) | MDA detection via TBA assay | Forms colored complex with MDA | Protect from light; prepare fresh solutions |
| 2,4-Dinitrophenylhydrazine (DNPH) | Carbonyl compound derivatization | Forms hydrazone derivatives with aldehydes | Purify by recrystallization for better results |
| Perfluorophenylhydrazine (PFPH) | GC-MS analysis of MDA | Derivatives MDA for enhanced volatility and detection | Use deuterated internal standards for quantification |
| Butylated Hydroxytoluene (BHT) | Sample preparation | Antioxidant to prevent artifactual oxidation during analysis | Add to extraction solvents (0.005-0.01%) |
| Deuterated Internal Standards | Quantitative mass spectrometry | Correct for analyte loss during sample preparation | Use MDA-d₂, 4-HNE-d₁₁, deuterated oxysterols |
| Solid-Phase Extraction Cartridges | Sample cleanup | Remove interfering matrix components | C18 for reverse phase; silica for normal phase |
| Silylation Reagents | Oxysterol analysis | Increase volatility for GC analysis | BSTFA with 1% TMCS commonly used |
Advanced Technical Considerations
When analyzing lipid oxidation biomarkers in complex food matrices, several technical considerations are essential for obtaining accurate and reproducible results:
Matrix Effects: Complex food matrices can significantly interfere with biomarker quantification. Use matrix-matched calibration standards or the standard addition method to compensate for these effects [89] [88].
Artifact Formation: Lipid oxidation can continue during sample preparation unless appropriate precautions are taken. Always include antioxidants like BHT in extraction solvents and work under inert atmosphere when possible [89] [90].
Comprehensive Profiling: Rather than analyzing single biomarkers, consider comprehensive approaches that measure multiple oxidation products simultaneously to obtain a complete picture of the oxidation status [88].
Method Validation: Rigorously validate analytical methods for each food matrix, including determination of accuracy, precision, recovery, limit of detection, and limit of quantification [89] [88].
The comprehensive analysis of lipid oxidation biomarkers—MDA, 4-HNE, and oxysterols—provides critical insights into the mechanisms of food rancidity and oxidative deterioration. The continuous advancement in analytical technologies, particularly the development of chromatographic-mass spectrometric methods with improved sensitivity and specificity, has significantly enhanced our ability to accurately quantify these biomarkers in complex food matrices. The selection of appropriate analytical methods should be guided by the specific research objectives, considering factors such as required sensitivity, specificity, throughput, and available resources.
Future research directions in this field should focus on the development of standardized analytical methods for biomarker quantification across different food matrices, investigation of the kinetics of formation and degradation of these biomarkers under various processing and storage conditions, and comprehensive assessment of the health implications of dietary exposure to these oxidation products. Additionally, the integration of multiple biomarker analysis rather than reliance on single markers will provide a more comprehensive assessment of lipid oxidation status in food systems, enabling better prediction of shelf-life, evaluation of antioxidant efficacy, and assessment of potential health risks associated with consumption of oxidized lipids.
Correlating Analytical Results with Sensory and Biological Outcomes
The integration of analytical chemistry, sensory evaluation, and biological measurement is paramount for comprehensively understanding food quality, particularly within the context of lipid oxidation and rancidity development. This technical guide details the methodologies and frameworks for establishing robust correlations between instrumental data, human perception, and physiological responses. It provides researchers and drug development professionals with validated experimental protocols, data presentation standards, and visualization tools to advance mechanistic studies in food chemistry and related health impacts.
Lipid oxidation is a primary cause of food spoilage, initiating a complex cascade of chemical and physical changes that profoundly affect food quality, nutritional value, and safety [4]. This process involves the autoxidation of unsaturated fatty acids, leading to the formation of free radicals and hydroperoxides, which subsequently degrade into a wide array of secondary products, including aldehydes, ketones, and alcohols [4]. These volatile compounds are responsible for the characteristic rancid odors and flavors and can induce protein oxidation and aggregation, further altering food's physicochemical and nutritional properties [4].
Understanding the full impact of lipid oxidation requires a multi-faceted approach that moves beyond mere analytical quantification. This whitepaper outlines a structured methodology for correlating analytical results from lipid oxidation studies with sensory outcomes (human perception of taste, aroma, and texture) and biological outcomes (measurable physiological responses in consumers). This integrative strategy is essential for elucidating the complete mechanism of food rancidity and its implications for human health and product development.
Analytical Foundations: Measuring Lipid Oxidation
A critical first step is the accurate quantification of lipid oxidation products. The choice of method depends on whether primary or secondary oxidation products are being measured.
Table 1: Key Assessment Methods for Lipid Oxidation
| Target | Method | Principle | Application Notes |
|---|---|---|---|
| Primary Products | Peroxide Value (PV) | Quantifies hydroperoxides via iodometric or ferric thiocyanate methods [4]. | Highly sensitive; requires precautions to prevent oxygen interference and hydroperoxide decomposition, especially in ground meat during long-term storage [4]. |
| Conjugated Diene Analysis | Measures diene conjugation at 233 nm [4]. | Low-cost and convenient for early-stage oxidation in polyunsaturated fatty acids; less effective for detecting small conjugated dienes and depends on lipoprotein composition and size [4]. | |
| Secondary Products | Thiobarbituric Acid Reactive Substances (TBARS) | Detects malondialdehyde (MDA) and other secondary oxidation products at 532 nm [4]. | Common for meat, fish, and edible insects; results can be influenced by other food components [4]. |
| Chromatography & Fluorometry | Gas Chromatography (GC) or High-Performance Liquid Chromatography (HPLC) coupled with mass spectrometry or fluorescence detection [4]. | Highly sensitive, fast, and accurate for specific volatile compounds like aldehydes; more costly and requires specialized equipment [4]. | |
| Sensory Analysis | Human evaluation of off-flavors and rancidity [4]. | Provides overall quality assessment for solid and liquid foods; limited by panelist training and temporal changes [4]. | |
| Volatile Aroma Compounds | Solid-Phase Microextraction (SPME) / GC-Olfactometry (GCO) | Extraction and separation of volatiles with olfactory detection to identify key aroma-active compounds [92]. | Critical for linking specific chemical compounds to sensory perception (aroma) [92]. |
| Electronic Nose | Array of sensors to characterize overall aroma profile [92]. | Can provide a rapid fingerprint of volatile compounds [92]. |
Sensory Outcomes: Bridging Chemistry and Perception
Sensory evaluation translates analytical data into human experience. The formation of aroma compounds—aldehydes, acids, esters, terpenes, pyrazines, and furans—is a direct result of lipid oxidation and other processes like cooking and fermentation [92]. These volatiles are key determinants of consumer acceptance [92].
A powerful tool for quantifying subjective sensory experiences is the Visual Analog Scale (VAS). In a model experiment analyzing strawberry taste, participants ate samples and evaluated perceptions using a VAS, providing quantitative data on attributes like sweetness, sourness, and overall palatability [93]. This data is crucial for correlation with concurrent analytical and biological measurements.
Biological Outcomes: Measuring Physiological Responses
Biological outcomes provide an objective, physiological dimension to complement sensory data. Functional Near-Infrared Spectroscopy (fNIRS) is a non-invasive brain imaging technique that measures blood flow in the cortical regions, offering insights into the neural correlates of taste perception [93].
Experimental Protocol: fNIRS for Taste Perception [93]
- Participants: Recruit healthy individuals without gustatory or olfactory disorders. Obtain ethical approval and informed consent.
- Setup: Participants are seated in a controlled environment (e.g., constant temperature of 23°C) and fitted with an fNIRS headset.
- Procedure: The experiment follows a block design:
- Rest Period (300 s): Baseline measurement with participants relaxed and eyes open.
- Stimulus Viewing (15 s): Participants view the food item.
- Rest Period (45 s): Baseline recovery.
- Ingestion Period: Participants take the food into their mouth, bite, and swallow immediately. fNIRS data is recorded during this phase.
- Rest Period (80 s): Post-ingestion baseline recovery.
- Evaluation Period: Participants complete VAS and other questionnaires on taste perception.
- Mouth Rinse: Participants rinse with pure water to neutralize the palate before the next trial.
- Data Analysis: fNIRS features (e.g., changes in oxygenated hemoglobin levels) calculated during the ingestion period are used for correlation analysis with VAS scores and analytical data.
Correlation Analysis: Integrating Multimodal Data
To discover potential relationships that may not be revealed by simple correlation analysis, Canonical Correlation Analysis (CCA) is a potent multivariate method [93]. CCA projects different data types (e.g., analytical, sensory, and biological features) into a common latent space to maximize their correlations.
For instance, CCA can be used to:
- Verify the correlation between fNIRS brain activity and VAS taste scores.
- Identify which questionnaire factors (e.g., cultural preferences) or which individual food information parameters (e.g., sugar-to-acid ratio) have the highest cross-loading with brain activity, suggesting a potential relationship with taste [93].
This approach can uncover latent connections, such as the influence of cultural factors or the specific role of acidity and sugar content on perceived taste and its corresponding neurological signature [93].
The following diagram illustrates the integrated experimental workflow from data acquisition to correlation analysis.
The Scientist's Toolkit: Essential Research Reagents and Materials
This section details key reagents and materials essential for conducting experiments in this field.
Table 2: Key Research Reagent Solutions
| Item | Function/Application |
|---|---|
| 2,2'-Azobis(2-amidinopropane) dihydrochloride | A radical initiator used in model systems to induce protein oxidation and aggregation, simulating the effects of lipid oxidation products [4]. |
| Thiobarbituric Acid | Reactive compound used in the TBARS assay to quantify malondialdehyde (MDA), a key secondary product of lipid oxidation [4]. |
| Potassium Iodide | Used in the iodometric titration for the Peroxide Value (PV) test, where it reacts with hydroperoxides to liberate iodine [4]. |
| Solid-Phase Microextraction (SPME) Fibers | Used for the headspace extraction and concentration of volatile aroma compounds from food samples prior to analysis by GC-MS [92]. |
| Solvent-Assisted Flavor Evaporation (SAFE) Apparatus | Used for the careful distillation and isolation of volatile flavor compounds from complex food matrices, minimizing artifact formation [92]. |
| fNIRS Equipment | Functional Near-Infrared Spectroscopy system, including a headset with light sources and detectors, for non-invasive measurement of brain activity related to sensory perception [93]. |
| Visual Analog Scale (VAS) | A psychometric response tool (e.g., a continuous line) used to obtain quantitative measures of subjective sensory perceptions like taste intensity and liking [93]. |
Data Integration and Visualization Workflow
The final stage involves synthesizing data from all three domains to build a comprehensive model of how lipid oxidation affects sensory perception and biological response. The following diagram maps the logical relationships between the core concepts and measurement outcomes discussed in this guide, illustrating the pathway from the initial stimulus to the final correlated understanding.
In Vitro and In Vivo Models for Assessing the Health Effects of Oxidized Lipids
Oxidized lipids, formed during food processing, storage, and digestion, significantly impact food quality and human health. Within the broader context of lipid oxidation and food rancidity research, understanding their biological effects is crucial for developing healthier food products and therapeutic interventions. This technical guide comprehensively outlines established in vitro and in vivo models for evaluating the health effects of these compounds, providing researchers with detailed methodologies, applications, and analytical frameworks. The formation of oxidized lipids in foods is an inevitable consequence of their exposure to oxygen, light, and heat, leading to rancidity and degradation of nutritional quality [3]. Beyond affecting food shelf-life and sensory properties, these compounds are increasingly recognized for their biological activities, which can range from pro-inflammatory and cytotoxic effects to potential signaling roles. This document serves as an essential resource for scientists and drug development professionals seeking to implement robust, standardized models for assessing the multifaceted health impacts of oxidized lipids, thereby bridging the gap between food chemistry and pathophysiological outcomes.
In Vitro Models
In vitro systems provide controlled, reproducible platforms for investigating the initial biological interactions and cytotoxic effects of oxidized lipids. These models are invaluable for mechanistic studies and high-throughput screening.
Simulated Gastrointestinal Digestion Models
The INFOGEST protocol is a widely adopted standardized method for simulating human gastrointestinal digestion in vitro, particularly useful for studying lipid oxidation during digestion and the effect of dietary components.
Detailed Protocol (INFOGEST 2.0 with modifications) [94]:
- Sample Preparation: Homogenize 5 g of cooked meat sample (or other test food) with 12.5 mL of a test beverage or 9.4 g of a test salad.
- Oral Phase: Add 5 mL of simulated saliva juice (containing α-amylase) to the mixture. Homogenize and incubate for 5 minutes at 37°C in a shaking water bath.
- Gastric Phase: Introduce 12 mL of simulated gastric juice (containing pepsin). Adjust the pH to 3.0 if necessary. Incubate for 30 minutes at 37°C with continuous shaking.
- Intestinal Phase: Add 5 mL of bile solution and 10 mL of simulated duodenal juice (containing pancreatin and lipase). Adjust the pH to 7.0. Incubate for 2 hours at 37°C with shaking.
- Termination and Analysis: Halt the reaction by adding 10% Trichloroacetic Acid (TCA). Centrifuge the mixture at 10,000 rpm for 10 minutes at 22°C. Filter the supernatant through a 0.45 μm filter for subsequent analysis (e.g., HPLC for malondialdehyde (MDA), glyoxal (GO), and methylglyoxal (MGO)).
Table 1: Key Markers and Analytical Methods for Lipid Oxidation in Digestion Models
| Marker | Significance | Common Analytical Method |
|---|---|---|
| Malondialdehyde (MDA) | Secondary product of lipid peroxidation; indicates oxidative stress [94] [3]. | HPLC with thiobarbituric acid (TBA) derivatization [94]. |
| Glyoxal (GO) | Reactive dicarbonyl compound; precursor for Advanced Glycation End-products (AGEs) [94]. | HPLC [94]. |
| Methylglyoxal (MGO) | Highly reactive dicarbonyl compound; major precursor to AGEs [94]. | HPLC [94]. |
| Lipid Hydroperoxides | Primary oxidation products; unstable and decompose to secondary products [3]. | Peroxide Value (PV) assay (iodometric or ferric thiocyanate) [3]. |
| Conjugated Dienes | Indicate early-stage oxidation in polyunsaturated fatty acids [3]. | Spectrophotometry (234 nm) [3]. |
Cell Culture Models
Cell-based systems are used to assess the cytotoxicity, oxidative stress, and inflammatory responses induced by oxidized lipids.
Detailed Protocol for HepG2 Lipid Accumulation and Oxidative Stress Assay [95]:
- Cell Culture: Maintain HepG2 cells (human liver carcinoma cell line) in DMEM medium supplemented with 10% Fetal Bovine Serum (FBS) and 1% antibiotics at 37°C in a 5% CO₂ atmosphere.
- Cytotoxicity Screening (CCK-8 Assay):
- Seed cells in a 96-well plate (5 × 10³ cells/well) and incubate for 24 hours.
- Treat with a concentration gradient of the test substance (e.g., 0-500 μg/mL of exosomes or oxidized lipid extracts) for 24 hours.
- Add CCK-8 reagent and incubate for 2 hours. Measure absorbance at 450 nm to determine cell viability and the non-cytotoxic concentration range.
- Induction of Lipid Accumulation:
- Induce a hyperlipidemic state by treating HepG2 cells with Oleic Acid (OA) to create a cellular steatosis model.
- Treatment and Analysis:
- Co-treat OA-induced cells with the test compound (e.g., potential therapeutic exosomes or antioxidants).
- Measure Lipid Parameters: Extract cellular lipids and quantify Triglycerides (TG), Total Cholesterol (TC), LDL-C, and HDL-C using commercial enzymatic kits.
- Assess Oxidative Stress:
- Reactive Oxygen Species (ROS): Use a fluorescent probe (e.g., DCFH-DA) and measure fluorescence.
- Antioxidant Enzymes: Measure Superoxide Dismutase (SOD) activity and Total Antioxidant Capacity (T-AOC) via colorimetric kits.
- Lipid Peroxidation: Quantify Malondialdehyde (MDA) levels as a marker of oxidative damage [95].
HepG2 Assay Workflow: This diagram outlines the key steps in using HepG2 cells to evaluate the effects of test compounds on OA-induced lipid accumulation and oxidative stress.
In Vivo Models
In vivo models are essential for understanding the systemic effects of oxidized lipids, including metabolic dysregulation, tissue inflammation, and the efficacy of therapeutic interventions in a whole-organism context.
High-Fat Diet (HFD)-Induced Hyperlipidemia Mouse Model
This model is a cornerstone for studying obesity, dyslipidemia, insulin resistance, and related metabolic disorders.
- Animals and Grouping: Use C57BL/6J mice (or similar strain). House at a controlled temperature (22±2°C) with a 12/12-hour light/dark cycle. After acclimatization, randomly assign mice into groups (e.g., Control diet, High-Fat Diet (HFD), HFD + intervention).
- Diet and Intervention:
- Control Group: Feed a standard chow diet (~10-15% kcal from fat).
- HFD Group: Feed a high-fat diet (typically 45-60% kcal from fat) for 8-12 weeks to induce hyperlipidemia and obesity.
- Intervention Group: Administer the test compound (e.g., herbal phenolic formula [96] or exosomes [95]) via oral gavage or diet mixing concurrently with the HFD feeding for the study duration.
- Monitoring: Weekly record of body weight and food intake.
- Sample Collection and Analysis:
- At endpoint, collect blood samples via cardiac puncture after fasting. Centrifuge to isolate plasma or serum.
- Euthanize animals and dissect key tissues (liver, adipose tissue, aorta). Weigh tissues and preserve for histological and molecular analysis.
- Key Outcome Measures:
- Plasma Lipid Profile: Quantify TC, TG, LDL-C, and HDL-C using enzymatic colorimetric assays.
- Glycemic Control: Measure fasting blood glucose and perform an oral glucose tolerance test (OGTT).
- Oxidative Stress Markers: Assess plasma and tissue levels of MDA, SOD, and T-AOC [95].
- Liver Lipid Analysis: Quantify hepatic TG and TC content.
- Histopathology: Examine liver sections (e.g., H&E staining) for steatosis, ballooning, and inflammation. Analyze adipose tissue for adipocyte hypertrophy [96] [97].
Table 2: Primary Outcome Measures in HFD Mouse Models
| Parameter Category | Specific Measures | Technique/Method |
|---|---|---|
| Systemic Metabolism | Body Weight, Adipose Tissue Weight, Fasting Blood Glucose | Gravimetric, Glucometer, OGTT |
| Plasma Lipid Profile | Total Cholesterol (TC), Triglycerides (TG), LDL-C, HDL-C | Enzymatic colorimetric assays |
| Oxidative Stress | Malondialdehyde (MDA), Superoxide Dismutase (SOD), Total Antioxidant Capacity (T-AOC) | Commercial kits (colorimetric/fluorometric) |
| Organ Damage & Morphology | Liver Steatosis, Adipocyte Hypertrophy | Histology (H&E staining), Tissue lipid extraction |
HFD Mouse Model Workflow: This diagram illustrates the experimental flow for a dietary intervention study in a high-fat diet mouse model, from group assignment to final analysis.
Integration with In Silico Approaches
Complementing in vivo findings with in silico methods, such as molecular docking, can predict the interactions between bioactive compounds and molecular targets, providing mechanistic insights.
Methodology [96]:
- Ligand Preparation: Obtain 3D structures of identified bioactive compounds (e.g., rosmarinic acid, naringenin from an herbal formula) from databases like PubChem. Energy-minimize the structures.
- Target Selection: Select protein targets involved in lipid and cholesterol metabolism (e.g., HMG-CoA reductase, PPARα/γ, PCSK9).
- Molecular Docking: Perform docking simulations using software like AutoDock Vina to predict binding affinities (kcal/mol) and binding poses (interacting amino acids) of the bioactives to the target proteins.
- Validation: Correlate strong in silico binding predictions with observed in vivo effects (e.g., plasma cholesterol-lowering linked to HMG-CoA reductase binding).
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagent Solutions for Oxidized Lipid Research
| Reagent / Material | Function / Application | Example Use Case |
|---|---|---|
| Pepsin, Pancreatin, Lipase, Bile Salts | Key enzymes and salts for simulating gastrointestinal digestion in the INFOGEST model. | In vitro digestion of meat to study lipid oxidation and the protective effects of antioxidants [94]. |
| Oleic Acid (OA) | A free fatty acid used to induce lipid accumulation and steatosis in hepatocyte cell cultures. | Creating an in vitro model of hyperlipidemia in HepG2 cells [95]. |
| Malondialdehyde (MDA) Assay Kit | Quantifies MDA, a key secondary product of lipid peroxidation, as a marker of oxidative stress. | Measuring oxidative damage in cell cultures, plasma, or tissues [94] [95]. |
| SOD & T-AOC Assay Kits | Measures the activity of antioxidant defense systems (Superoxide Dismutase and Total Antioxidant Capacity). | Evaluating the antioxidant efficacy of a test compound in vivo or in vitro [95]. |
| Enzymatic Colorimetric Kits (TC, TG, LDL-C, HDL-C) | For the quantitative analysis of lipid species in plasma, serum, or tissue homogenates. | Determining the plasma lipid profile in mouse models of hyperlipidemia [96] [95]. |
| High-Fat Diet (HFD) | A defined rodent diet high in fat content (45-60% kcal) used to induce obesity and metabolic syndrome. | Establishing an in vivo model for studying dyslipidemia and the therapeutic effects of compounds [96] [95]. |
| CCK-8 Cell Viability Kit | A colorimetric assay used to determine cell proliferation and cytotoxicity. | Screening the safe dosage range of test compounds on HepG2 or other cell lines [95]. |
Lipid oxidation is a paramount cause of quality deterioration in foods, leading to nutrient loss, development of rancid off-flavors, and formation of potentially harmful compounds. This complex process manifests differently across various food matrices, influenced by their distinct compositions, physical structures, and environmental exposures. Understanding these matrix-specific pathways is crucial for developing targeted strategies to mitigate oxidation and extend shelf life. This review provides a comprehensive technical analysis of oxidation mechanisms in four critical food systems: bulk oils, oil-in-water emulsions, meat products, and low-moisture foods. Within the broader context of food rancidity research, we examine how interfacial phenomena, compositional factors, and water activity modulate oxidative stability, supported by comparative experimental data and detailed methodologies for assessing oxidation endpoints across these diverse systems.
Fundamental Mechanisms of Lipid Oxidation
Lipid oxidation proceeds via a free radical chain reaction comprising three core stages: initiation, propagation, and termination. In the initiation phase, reactive oxygen species abstract hydrogen from unsaturated fatty acids (LH), forming lipid alkyl radicals (L•). During propagation, these radicals rapidly react with molecular oxygen to form lipid peroxyl radicals (LOO•), which subsequently attack other fatty acids to generate lipid hydroperoxides (LOOH) and new alkyl radicals. Termination occurs when radicals combine to form non-radical products [4].
The specific hydroperoxides formed depend on the unsaturated fatty acid substrate. Oleic acid (C18:1) generates four hydroperoxide isomers, while linoleic acid (C18:2) and α-linolenic acid (C18:3) form two and four specific hydroperoxide isomers respectively due to the stabilization of pentadienyl radicals [4]. These primary oxidation products are odorless but break down into volatile secondary oxidation products—including aldehydes, ketones, alcohols, and hydrocarbons—responsible for rancid odors and flavors. The particular volatile profile depends on the precursor fatty acids; for example, linoleic acid oxidation predominantly yields hexanal, while α-linolenic acid produces 2,4-heptadienal [98].
Lipid Oxidation Pathway Diagram
Oxidation Across Food Matrices
Bulk Oils
In bulk oil systems, the homogeneous lipid phase allows relatively unrestricted movement of reactants and catalysts. Mealworm oil, rich in polyunsaturated fatty acids (PUFAs), demonstrates characteristic oxidation patterns under thermal stress. During heating at 180°C, the acid value increases rapidly within the initial 30 minutes, indicating hydrolytic cleavage of fatty acids from triglycerides. The peroxide value similarly spikes early, reflecting hydroperoxide accumulation, while the p-anisidine value increases gradually then sharply after 4 hours, marking significant secondary oxidation product formation [98].
Table 1: Oxidation Indices in Mealworm Oil During Thermal Treatment at 180°C [98]
| Heating Time | Acid Value (mg KOH/g) | Peroxide Value (meq/kg) | p-Anisidine Value |
|---|---|---|---|
| 0 minutes | 1.2 | 2.5 | 12.1 |
| 30 minutes | 3.8 | 15.2 | 15.8 |
| 1 hour | 4.5 | 22.4 | 18.3 |
| 2 hours | 5.2 | 28.7 | 22.6 |
| 4 hours | 6.1 | 32.5 | 25.4 |
| 8 hours | 8.3 | 35.8 | 45.2 |
Oil-in-Water Emulsions
Emulsions present a fundamentally different oxidative environment where interfacial phenomena dominate oxidation kinetics. The large oil-water interface facilitates interactions between lipid substrates and aqueous phase pro-oxidants. In oil-in-water (O/W) emulsions, the composition and structure of the interfacial membrane critically influence stability. Emulsifier type determines initial oxidative stability, with proteins forming viscoelastic films that provide both steric hindrance and electrostatic repulsion against pro-oxidants [99].
Recent research explores High Internal Phase Pickering Emulsions (HIPPEs) stabilized by ultrasound-modified almond protein isolate (UAPI) particles as fat substitutes in meat products. At 300W ultrasonication, UAPI particles achieve optimal surface hydrophobicity and emulsification activity, producing emulsions with minimal droplet size (30.49 μm) and enhanced oxidative stability. When these HIPPEs replace pork backfat in sausages, they reduce thiobarbituric acid-reactive substances (TBARS) values, indicating suppressed lipid oxidation [100].
Meat Products
Meat matrices present complex oxidation dynamics due to the coexistence of lipids, proteins, heme pigments, and transition metals. Reformulation strategies to improve nutritional profiles often increase PUFAs, creating oxidation challenges. Replacing pork backfat with nut oil emulsions in Spanish sobrasada significantly improves the fatty acid profile—reducing saturated fats by up to 64% and increasing PUFAs by 226%—but necessitates careful oxidation management [101].
Table 2: Fatty Acid Profile Changes in Reformulated Meat Products [101] [102]
| Product Type | Treatment | SFA (%) | MUFA (%) | PUFA (%) | n-6/n-3 Ratio | TBARS (mg MDA/kg) |
|---|---|---|---|---|---|---|
| Spanish Sobrasada | Control (pork fat) | 38.9 | 50.1 | 11.0 | 12.5 | 0.45 |
| 100% Almond oil emulsion | 14.0 | 75.8 | 10.2 | 9.8 | 0.52 | |
| 100% Walnut oil emulsion | 16.5 | 28.4 | 55.1 | 4.2 | 0.61 | |
| Chicken Frankfurters | Control (pork fat) | 41.2 | 45.3 | 13.5 | 14.8 | 0.28 |
| Linseed oil emulsion | 12.7 | 31.5 | 55.8 | 1.2 | 0.89 | |
| Walnut oil emulsion | 13.5 | 28.4 | 58.1 | 3.5 | 0.51 | |
| Algal oil emulsion | 14.1 | 25.3 | 60.6 | 0.4 | 1.24 |
In meat products, lipid and protein oxidation proceed synergistically. Lipid-derived free radicals and secondary oxidation products promote protein oxidation, leading to protein aggregation through disulfide bond formation, amino acid modification, and carbonyl generation. This negatively impacts protein functionality, digestibility, and sensory quality [4].
Low-Moisture Foods
Low-moisture foods like cereal flours exhibit distinct oxidation patterns governed by enzymatic activity and water mobility. Pearl millet flour, with high PUFA content (5-6%), is particularly susceptible to enzymatic rancidity mediated by indigenous lipases and lipoxygenases. During storage, lipase hydrolyzes triglycerides into free fatty acids, while lipoxygenase oxidizes PUFAs to hydroperoxides, which decompose to volatile off-flavor compounds [9].
Metabolomic profiling of rancid pearl millet flour reveals pronounced metabolic shifts, including elevated levels of lipid degradation products (3-oxotetradecanoyl-CoA), free fatty acids, and pigments like chlorophyllide b. Enzymatic assays show storage-dependent increases in lipase, lipoxygenase, peroxidase, and polyphenol oxidase activities, correlating strongly with rancidity indices (acid value, peroxide value) [9]. The landrace Chadhi Bajri demonstrates greater oxidative resistance than the hybrid Pusa-1201, accumulating protective metabolites like quercetin 3-sulfate and rosmarinate [9].
Experimental Protocols for Oxidation Assessment
Comprehensive Oxidation Analysis Workflow
Standardized Chemical Analyses
Peroxide Value (PV) - AOCS Cd 8-53 [98] Principle: Measures hydroperoxides as primary oxidation products. Protocol: Dissolve 1g oil in 25mL acetic acid:chloroform (3:2). Add 1mL saturated KI, stand in dark for 10min. Add 30mL water and starch indicator. Titrate with 0.01N Na₂S₂O₃ until blue color disappears. Calculate: PV = [(S-B) × N × 1000]/sample weight (g), where S = sample titre, B = blank titre, N = Na₂S₂O₃ normality.
p-Anisidine Value (AnV) - AOCS Cd 18-90 [98] Principle: Assesses secondary oxidation products (aldehydes). Protocol: Dissolve 0.5g oil in 25mL iso-octane, measure absorbance at 350nm (Ab). Mix 2.5mL solution with 1mL 0.25% p-anisidine in acetic acid, react 10min. Measure absorbance at 350nm (As). Calculate: AnV = [25 × (1.2As - Ab)]/sample weight (g).
Thiobarbituric Acid Reactive Substances (TBARS) [102] Principle: Quantifies malondialdehyde (MDA) as secondary oxidation marker. Protocol: Homogenize sample with 3M HCl solution. Add TBA reagent, heat 30min at 95°C. Cool, measure absorbance at 532nm. Calculate from standard curve.
Acid Value (AV) - AOCS Cd 3d-63 [98] Principle: Measures free fatty acids from hydrolytic rancidity. Protocol: Dissolve 5g oil in 100mL ether:ethanol (2:1). Titrate with 0.1N KOH using phenolphthalein indicator. Calculate: AV = [5.611 × (S-B) × N]/sample weight (g).
Advanced Analytical Techniques
LC-MS Metabolomic Profiling [9] Application: Comprehensive oxidation biomarker discovery. Protocol: Extract 50mg flour with 80% methanol, then chloroform. Combine extracts, dry under N₂, reconstitute in acetonitrile:isopropanol:water (65:30:5). Analyze using UHPLC-Orbitrap MS with C18 column (2.1×100mm, 1.7μm). Gradient elution with 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). Data processing with multivariate statistics (PLS-DA, VIP selection).
Volatile Compound Analysis by GC-MS [98] Application: Secondary oxidation product profiling. Protocol: Headspace SPME sampling. Extract volatiles using DVB/CAR/PDMS fiber, 40°C for 30min. GC separation with DB-5MS column, temperature programming. MS detection in EI mode, 40-350m/z. Identify compounds using NIST library, quantify with internal standards.
The Scientist's Toolkit: Key Research Reagents and Materials
Table 3: Essential Reagents and Materials for Oxidation Research
| Category | Specific Reagents/Materials | Research Application | Key Function |
|---|---|---|---|
| Chemical Standards | Undecanoic acid, 2-chlorophenylalanine, D4-glutamic acid [9] | LC-MS metabolomics | Internal standards for quantification |
| 37 FAME mix, cis-11-vaccenic acid methyl ester [98] | GC-MS fatty acid analysis | Fatty acid profile determination | |
| 2,2-Diphenyl-1-picrylhydrazyl (DPPH) [100] | Antioxidant capacity assay | Free radical scavenging assessment | |
| Analytical Reagents | p-Anisidine, thiobarbituric acid [98] | Secondary oxidation analysis | Aldehyde quantification |
| Potassium iodide, sodium thiosulfate [98] | Peroxide value determination | Hydroperoxide measurement | |
| BF₃-methanol solution [98] | Fatty acid methylation | FAME preparation for GC | |
| Emulsification Systems | Inulin fibers (Fibruline) [102] | Emulsion gel preparation | Oil structuring, fat replacement |
| Soy protein isolate, whey protein [99] | Interface stabilization | Emulsion formation and stabilization | |
| Guar gum, carrageenan [101] | Polysaccharide emulsifiers | Thickening, stabilization | |
| Model Oil Systems | Linseed oil, walnut oil, algal oil [102] | PUFA-rich oxidation models | Oxidation substrate studies |
| Mealworm oil (Tenebrio molitor) [98] | Insect oil oxidation model | Alternative lipid source stability |
The comparative analysis of oxidation across food matrices reveals both universal chemical principles and matrix-specific manifestations. Bulk oils follow classical autoxidation kinetics, while emulsions introduce interfacial phenomena that accelerate oxidation. Meat products exhibit complex lipid-protein co-oxidation dynamics, and low-moisture foods experience enzyme-mediated rancidity pathways. This understanding enables targeted stabilization strategies: interfacial engineering for emulsions, ingredient selection and packaging for meats, and enzyme inactivation for low-moisture systems. Future research should further elucidate molecular-level interactions at food interfaces and develop integrated preservation approaches that address matrix-specific oxidation mechanisms while maintaining nutritional and sensory quality. The continued advancement of analytical methodologies, particularly multi-omics approaches, will provide deeper insights into oxidation pathways across diverse food systems.
The consumption of dietary oxidized lipids, generated during the thermal processing of cooking oils, has emerged as a significant modifiable risk factor in the pathogenesis of chronic inflammatory diseases, particularly atherosclerosis. This whitepaper synthesizes current mechanistic understandings of how lipid oxidation products (LOPs) derived from dietary sources promote endothelial dysfunction, drive pro-inflammatory signaling cascades, and accelerate plaque development. Within the broader context of lipid oxidation and food rancidity research, we delineate the molecular pathways linking these dietary compounds to cardiovascular pathology, summarize key experimental data, and provide detailed methodologies for investigating these mechanisms. The presented framework aims to equip researchers and drug development professionals with the tools and insights necessary to advance therapeutic interventions targeting this critical pathway.
Atherosclerosis, a chronic inflammatory vascular disease characterized by the accumulation of lipid-rich plaques in the arterial intima, remains the leading underlying cause of cardiovascular mortality worldwide [103] [104]. While endogenous lipid oxidation within the arterial wall has long been recognized as a central driver of disease, a growing body of evidence implicates dietarily derived oxidized lipids as potent initiators and accelerants of this pathology [105]. When cooking oils, particularly those rich in polyunsaturated fatty acids (PUFAs), are subjected to high temperatures or repeated heating—a common practice in both food industry and domestic settings—they undergo extensive oxidative degradation, forming a complex mixture of LOPs including hydroperoxides, reactive aldehydes, and oxidized polymers [105] [55].
These exogenous LOPs are absorbed through the gastrointestinal tract, incorporated into chylomicrons, and enter the systemic circulation, where they contribute to the pool of pro-inflammatory and pro-atherogenic mediators [105]. This direct pathway bridges the gap between food quality research and chronic disease mechanisms, positioning dietary lipid oxidation not merely as a matter of food rancidity and sensory deterioration, but as a critical component in the onset and progression of atherosclerosis. The following sections provide a detailed examination of the mechanisms, experimental evidence, and research methodologies defining this field.
Pathophysiological Mechanisms and Signaling Pathways
From Dietary Intake to Vascular Inflammation
The journey of dietary oxidized lipids from the frying pan to the atherosclerotic plaque involves a well-defined sequence of events, culminating in endothelial dysfunction and inflammatory cell recruitment.
- Absorption and Systemic Circulation: Upon consumption of foods prepared with thermally oxidized oils, LOPs are absorbed intestinally. They are packaged into chylomicron remnants, which transport them into the circulatory system [105]. This process introduces a significant systemic load of pro-oxidant and pro-inflammatory species.
- Endothelial Activation and Lipoprotein Retention: A key initiating event in atherosclerosis, as per the "response-to-retention" hypothesis, is the subendothelial retention of apolipoprotein B (ApoB)-containing lipoproteins [106]. Dietary LOPs exacerbate this by promoting endothelial dysfunction. They activate endothelial cells, increasing the expression of adhesion molecules such as VCAM-1 and ICAM-1, which facilitate the firm adhesion of circulating monocytes to the vascular endothelium [107] [108]. Simultaneously, ApoB-containing lipoproteins, including those carrying dietary LOPs, bind to proteoglycans in the arterial intima, becoming trapped [106].
- Foam Cell Formation: Once retained, these lipoproteins undergo further oxidative modifications. Monocytes that have migrated into the subendothelial space differentiate into macrophages, which then avidly take up the oxidized lipids via scavenger receptors (e.g., SR-A, CD36) [107] [104]. The uncontrolled uptake of oxidized lipids leads to the formation of lipid-laden foam cells—the hallmark cellular component of early atherosclerotic lesions [103] [104].
Key Lipid Oxidation Products and Their Signaling Cascades
The primary toxicological drivers of dietary lipid-induced inflammation are reactive aldehydes generated during the peroxidation of PUFAs. The most extensively studied are malondialdehyde (MDA) and 4-hydroxy-2-nonenal (4-HNE) [105].
Malondialdehyde (MDA) is a highly reactive dialdehyde that readily forms covalent adducts with proteins and DNA, a process that contributes to cellular dysfunction and mutagenesis [105]. In the context of atherosclerosis, MDA-modified LDL is a key ligand for scavenger receptor uptake by macrophages, directly promoting foam cell formation [103]. Furthermore, MDA can promote the activation of the NLRP3 inflammasome, a multiprotein complex that orchestrates a potent inflammatory response. As illustrated in the pathway diagram below, MDA, along with other danger signals, can trigger NLRP3 inflammasome assembly, leading to the activation of caspase-1 and the subsequent maturation and secretion of pro-inflammatory cytokines IL-1β and IL-18 [105] [108].
4-Hydroxy-2-Nonenal (4-HNE), an α,β-unsaturated hydroxyalkenal, is notably more toxic than MDA due to its strong electrophilicity [105]. It readily reacts with thiol groups on proteins and other biomolecules, disrupting redox signaling and causing cytotoxicity. 4-HNE has been shown to be chemotactic for immune cells and can promote the synthesis of inflammatory cytokines, thereby amplifying local tissue injury and inflammatory responses within the nascent plaque [105].
The diagram below integrates these key players and pathways, mapping the journey from dietary intake to established plaque inflammation.
Quantitative Data and Experimental Evidence
Empirical data from both animal models and human studies provide compelling evidence for the role of dietary oxidized lipids in promoting inflammation and atherosclerosis. The following tables summarize key quantitative findings from this research.
Table 1: Effects of Feeding Thermally Oxidized Oils in Animal Models
| Animal Model | Intervention (Oil Type) | Key Inflammatory Findings | Oxidative Stress Markers | Primary Reference |
|---|---|---|---|---|
| Rats | Repeatedly heated palm oil | ↑ Expression of adhesion molecules (VCAM-1, ICAM-1) in aorta; Positive correlation with blood pressure | Not Specified | [105] |
| Rats | Repeatedly heated soy oil | ↑ Expression of adhesion molecules in aorta | Not Specified | [105] |
| Rats | Repeatedly heated virgin coconut oil (6 months) | ↑ Inflammatory markers; ↑ Thromboxane; ↓ Prostacyclin | Not Specified | [105] |
| Broiler chickens | Oxidized soybean oil (various levels) | ↑ Ileal IL-22 mRNA expression | ↑ MDA in intestine & liver; ↓ Total antioxidant capacity & SOD activity | [105] |
| Mice | Polar compounds from frying oil | Exacerbated experimental colitis; ↑ Inflammatory cytokines in colon and plasma | Not Specified | [105] |
Table 2: Key Lipid Oxidation Products and Their Pro-Atherogenic Effects
| Oxidation Product | Source / Formation | Measured Level Changes | Documented Pathogenic Effects | Primary Reference |
|---|---|---|---|---|
| Malondialdehyde (MDA) | Peroxidation of PUFAs (e.g., Arachidonic Acid) | Elevated in serum of hypertensive patients [105] | - Protein/DNA adduct formation- Activates NLRP3 inflammasome- Ligand for oxidized LDL uptake | [103] [105] |
| 4-Hydroxy-2-Nonenal (4-HNE) | Peroxidation of n-6 PUFAs | Elevated in oxidative stress conditions [105] | - Covalent modification of proteins, lipids, nucleic acids- Chemotactic for immune cells- Promotes cytokine synthesis | [105] |
| OxPL-apoB (Oxidized Phospholipids on ApoB) | Major carrier is Lipoprotein(a) [Lp(a)] | Baseline median: 26.5 nmol/L in OCEAN(a)-DOSE trial; >90% reduction with Olpasiran [109] | - Potent driver of inflammation & atherosclerosis- Activates endothelial cells | [109] [106] |
Recent clinical trials have further refined our understanding of this pathway. The OCEAN(a)-DOSE trial demonstrated that Olpasiran, a small interfering RNA that targets and reduces Lp(a) synthesis, achieved a dose-dependent and sustained reduction of up to ~93.7% in OxPL-apoB at 36 weeks [109] [110]. Interestingly, this profound reduction did not translate into significant changes in systemic inflammatory biomarkers like hs-CRP or hs-IL-6, suggesting that the atherogenicity of OxPL may be mediated through localized effects within the vessel wall rather than systemic inflammation, or that these particular biomarkers are not sensitive to changes in this specific pathway [109] [111].
Experimental Protocols and Methodologies
To investigate the link between dietary oxidized lipids and disease, robust and standardized experimental protocols are essential. The following section outlines key methodologies cited in the literature.
In Vivo Animal Feeding Studies
Objective: To evaluate the chronic systemic effects of consuming thermally oxidized oils on inflammation, oxidative stress, and early markers of atherosclerosis.
Protocol Details:
- Oil Preparation: Cooking oil (e.g., palm, soy, or virgin coconut oil) is subjected to controlled heating. A standard protocol involves heating at 180°C for 8-12 hours, often in cycles simulating repeated frying, to generate oxidized lipids [105].
- Animal Models and Diet Formulation: Common models include Spontaneously Hypertensive Rats (SHRs) or normotensive strains like Wistar-Kyoto rats. The test diet is formulated by mixing the heated oil (e.g., at 10-15% w/w) into a standard chow. Control groups receive chow blended with fresh oil [105].
- Study Duration: Feeding durations are typically chronic, ranging from several weeks to 6 months, to assess long-term pathological changes [105].
- Sample Collection and Analysis:
- Blood Pressure Monitoring: Serial blood pressure measurements are taken using tail-cuff plethysmography or telemetry [105].
- Tissue Collection: At sacrifice, blood, aorta, liver, and other organs are collected.
- Plasma/Sera Analysis: ELISA is used to quantify inflammatory cytokines (TNF-α, IL-1β, IL-6) and oxidative stress markers like MDA [105].
- Tissue Analysis: Aortic tissue is analyzed for gene and protein expression of adhesion molecules (VCAM-1, ICAM-1) via qRT-PCR and immunohistochemistry [105].
In Vitro Endothelial Cell Activation Assay
Objective: To directly assess the pro-inflammatory potential of lipid oxidation products on the vascular endothelium.
Protocol Details:
- Cell Culture: Human Umbilical Vein Endothelial Cells (HUVECs) are maintained in standard endothelial growth medium [105] [108].
- Treatment with LOPs: Cells are treated with purified aldehydes (e.g., 4-HNE or MDA) or extracts from thermally oxidized oils. A typical dose range is 1-100 µM for pure aldehydes, with exposure times from 6 to 24 hours [105].
- Analysis of Inflammatory Response:
- Cell Surface ELISA or Flow Cytometry: To quantify the increased expression of adhesion molecules (VCAM-1, ICAM-1, E-selectin) on the surface of activated HUVECs [107] [108].
- Monocyte Adhesion Assay: A functional assay where fluorescently labeled monocytes (e.g., THP-1 cell line) are co-cultured with treated HUVECs. The number of firmly adhered monocytes is quantified by fluorescence microscopy after washing away non-adherent cells [107].
The workflow for these core experiments is summarized in the following diagram:
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents and Resources for Investigating Dietary LOPs in Atherosclerosis
| Category / Item | Function / Specific Role | Example Application / Notes |
|---|---|---|
| Key Animal Models | ||
| Apoe-/- or Ldlr-/- Mice | Genetic models of hypercholesterolemia; develop robust atherosclerosis. | Gold-standard for studying plaque progression. [104] |
| Spontaneously Hypertensive Rats (SHRs) | Model for studying hypertension and associated vascular inflammation. | Useful for probing LOP effects on blood pressure. [105] |
| Cell Lines | ||
| Human Umbilical Vein Endothelial Cells (HUVECs) | Model for studying endothelial activation, adhesion molecule expression, and monocyte adhesion. | Primary cells with limited passages. [105] [108] |
| THP-1 Monocytic Cell Line | Human monocyte line; can be differentiated into macrophages. Used in adhesion and foam cell formation assays. | Differentiate with PMA for macrophage studies. [107] |
| Assay Kits & Reagents | ||
| MDA ELISA or TBARS Assay Kit | Quantifies malondialdehyde levels in plasma, serum, or tissue homogenates as a marker of lipid peroxidation. | Common colorimetric or fluorometric readouts. [105] |
| Cytokine ELISA Kits (TNF-α, IL-1β, IL-6, IL-18) | Measure levels of pro-inflammatory cytokines in cell culture supernatant or plasma. | Essential for quantifying inflammatory response. [105] [108] |
| Antibodies for VCAM-1, ICAM-1 | Detect protein expression of adhesion molecules via flow cytometry, Western blot, or immunohistochemistry. | Confirm endothelial activation in vitro and in vivo. [107] [108] |
| Key Chemical Reagents | ||
| 4-Hydroxy-2-Nonenal (4-HNE) | Pure, defined LOP for direct treatment in mechanistic in vitro studies. | Handle with care; highly reactive. Prepare fresh stocks. [105] |
| Malondialdehyde (MDA) | Pure standard for treatment and for use as a standard in assay calibration. | Often available as tetramethoxypropane (TMP), a stable precursor. [105] |
| Oxidized LDL (oxLDL) | Positive control for inducing foam cell formation in macrophage assays. | Commercially available from biological suppliers. [104] |
The body of evidence unequivocally links the consumption of dietary oxidized lipids to the exacerbation of inflammatory pathways that drive atherosclerosis. The mechanisms involve a cascade from systemic absorption and endothelial dysfunction to foam cell formation and plaque progression, driven by reactive aldehydes like MDA and 4-HNE. While lipid-lowering therapies like Olpasiran can dramatically reduce carriers of oxidized phospholipids, the disconnect between these reductions and systemic inflammatory biomarkers underscores the complexity of this pathway and the need for further research.
Future work should focus on:
- Identifying more sensitive and specific biomarkers that reflect arterial wall-specific inflammation related to LOPs.
- Elucidating the precise molecular mechanisms by which MDA and 4-HNE activate innate immune sensors like the NLRP3 inflammasome.
- Developing targeted therapeutic or dietary interventions that can specifically neutralize or prevent the formation of these reactive LOPs both in food and in vivo.
Integrating the principles of food chemistry, particularly lipid oxidation kinetics and antioxidant strategies, with cardiovascular pathophysiology provides a powerful, multidisciplinary approach to mitigating this modifiable risk factor for chronic disease.
Conclusion
The study of lipid oxidation bridges food science and human health, with clear mechanistic understanding enabling the development of effective analytical and stabilization strategies. The pathways of autoxidation and photo-oxidation dictate the profile of resulting hydroperoxides and secondary products, which can be precisely mapped using advanced techniques like HPLC-MS/MS. While antioxidants and optimized processing can control rancidity in food products, the secondary oxidation products remain a significant concern for human health, with evidence from animal models linking them to cellular damage, inflammation, and chronic diseases. Future research must focus on elucidating the precise dose-response relationships in humans, developing more sensitive biomarkers of exposure and effect, and creating novel delivery systems for natural antioxidants. For drug development, this underscores the importance of considering lipid oxidation not just in nutraceutical stability but also as a modifiable risk factor in disease pathogenesis, opening avenues for targeted therapeutic interventions.
References
- 1. Aged Butter part 2: the science of rancidity [https://nordicfoodlab.org/blog/2025/01/aged-butter-part-2-the-science-of-rancidity/]
- 2. Understanding Food Spoilage: Mechanisms, Shelf-Life ... [https://journals.stmjournals.com/rrjofst/article=2025/view=195721/]
- 3. Lipid oxidation in foods and its implications on proteins [https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2023.1192199/full]
- 4. Lipid oxidation in foods and its implications on proteins [https://pmc.ncbi.nlm.nih.gov/articles/PMC10307983/]
- 5. Rancidity in fats and oils: Considerations for analytical testing [https://ew-nutrition.com/us/rancidity-fats-oils-considerations-analytical-testing/]
- 6. Oxidative Rancidity Mechanisms & Food Stability [https://www.mpbio.com/us/oxidative-rancidity-mechanism?srsltid=AfmBOop5xuMGt1ktuaObtwviWDAO8dM_fJcxdMIDqluiCJZYOJpqhYln]
- 7. Integrated lipidomics and flavoromics approach elucidates the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12538410/]
- 8. Recent Advances in the Mechanisms of Quality Degradation ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11720141/]
- 9. Characterising the enzyme-driven metabolic shifts in rancid ... [https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2025.1691522/full]
- 10. Research Progress in the Rancidity Mechanism and ... [https://www.sciopen.com/article/10.7506/spkx1002-6630-20230621-171]
- 11. Autoxidation [https://en.wikipedia.org/wiki/Autoxidation]
- 12. Initiation Propagation Termination in Radical Reactions [https://www.chemistrysteps.com/initiation-propagation-termination-in-radical-reactions/]
- 13. Initiation, Propagation, Termination [https://www.masterorganicchemistry.com/2013/09/06/initiation-propagation-termination/]
- 14. Radical Reaction - an overview [https://www.sciencedirect.com/topics/engineering/radical-reaction]
- 15. Free Radicals in Chemical Biology [https://pmc.ncbi.nlm.nih.gov/articles/PMC3664958/]
- 16. Mechanisms of Photosensitized Lipid Oxidation and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6933592/]
- 17. Photooxidation - an overview [https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/photooxidation]
- 18. Rancidification [https://en.wikipedia.org/wiki/Rancidification]
- 19. Photo-Oxidation of Therapeutic Protein Formulations [https://pmc.ncbi.nlm.nih.gov/articles/PMC8779573/]
- 20. Analysis of docosahexaenoic acid hydroperoxide isomers ... [https://www.nature.com/articles/s41598-023-28514-2]
- 21. LC-MS/MS analysis of milk triacylglycerol hydroperoxide ... [https://www.sciencedirect.com/science/article/abs/pii/S0963996923014618]
- 22. Elucidating mechanisms of oxidative degradation in foods ... [https://portal.nifa.usda.gov/web/crisprojectpages/1025721-elucidating-mechanisms-of-oxidative-degradation-in-foods-and-developing-methods-for-its-detection-and-control.html]
- 23. Lipid Oxidation - an overview | ScienceDirect Topics [https://www.sciencedirect.com/topics/immunology-and-microbiology/lipid-oxidation]
- 24. Quantitative and Predictive Modelling of Lipid Oxidation in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7919052/]
- 25. Hydroperoxide - an overview [https://www.sciencedirect.com/topics/nursing-and-health-professions/hydroperoxide]
- 26. Lipid hydroperoxides in nutrition, health, and diseases - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC8062262/]
- 27. Comprehensive electrochemical and machine learning ... [https://www.sciencedirect.com/science/article/pii/S1452398124003419]
- 28. Food lipid oxidation and health [https://www.ifis.org/blog/food-lipid-oxidation-and-health]
- 29. Molecular Mechanisms Underlying Sensory and Chemical ... [https://www.mdpi.com/2304-8158/14/17/2967]
- 30. Lipid oxidation and lipidomic profiles of raw and thermal ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11894325/]
- 31. Revised Understanding of Lipid Oxidation Mechanisms [https://portal.nifa.usda.gov/web/crisprojectpages/1011866-revised-understanding-of-lipid-oxidation-mechanisms.html]
- 32. Analytical Methods for Lipid Oxidation and Antioxidant ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8533275/]
- 33. Relationship between changes in peroxide value and ... [https://grasasyaceites.revistas.csic.es/index.php/grasasyaceites/article/view/561]
- 34. Peroxide Value - an overview [https://www.sciencedirect.com/topics/food-science/peroxide-value]
- 35. Peroxide value [https://en.wikipedia.org/wiki/Peroxide_value]
- 36. Improving Prediction of Peroxide Value of Edible Oils ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8659081/]
- 37. A unifying approach to lipid oxidation in emulsions [https://www.sciencedirect.com/science/article/pii/S0963996922006792]
- 38. P-Anisidine Value - an overview [https://www.sciencedirect.com/topics/food-science/p-anisidine-value]
- 39. 2.6.3. p-Anisidine Value Measurement [https://bio-protocol.org/exchange/minidetail?id=10753836&type=30]
- 40. p-Anisidine Value (AnV) test in Fats and Oils [https://www.cdrfoodlab.com/cdrfoodlab/analyses/anisidine-value-oils-fats]
- 41. Thiobarbituric Acid - an overview [https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/thiobarbituric-acid]
- 42. Lipid oxidation in food science and nutritional health [https://www.sciencedirect.com/science/article/pii/S209624282300009X]
- 43. Analysis of docosahexaenoic acid hydroperoxide isomers ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9873796/]
- 44. Analysis of the trend of volatile compounds by HS-SPME ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9304473/]
- 45. Exploring the oxidative rancidity mechanism and changes ... [https://www.sciencedirect.com/science/article/pii/S2590157523005515]
- 46. Vegetable oil oxidation: Mechanisms, impacts on quality, ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12146556/]
- 47. Shade of Innovative Food Processing Techniques [https://www.mdpi.com/1420-3049/28/24/8138]
- 48. Study of the oxidative stability via Oxitest and Rancimat ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11629540/]
- 49. Oxidation stability of oils and fats – Rancimat method [https://www.metrohm.com/en/applications/ab-application-bulletins/ab-204.html]
- 50. 如何确定您的食用油是否变酸败? [https://www.metrohm.com/zh_cn/discover/blog/20-21/how-to-determine-if-your-edible-oils-are-rancid.html]
- 51. 使用Rancimat 方法测定氧化和热稳定性 [https://www.metrohm.com/zh_cn/products/stability-measurement/Stability-measurement-Rancimat-Thermomat.html]
- 52. Analytical Methods for Lipid Oxidation and Antioxidant ... [https://www.mdpi.com/2076-3921/10/10/1587]
- 53. A New Insight into the Weight Gain Method to Monitor and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11854536/]
- 54. Quantitative descriptive analysis and principal component ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4837733/]
- 55. Lipid Oxidation Pathways in Food Systems: Insights into ... [https://www.frontiersin.org/research-topics/71932/lipid-oxidation-pathways-in-food-systems-insights-into-antioxidant-strategies-and-advanced-detection-methodsundefined]
- 56. Free radicals and their impact on health and antioxidant ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11760946/]
- 57. A Comparative Study on Oxidative Stability of Lipids in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12382763/]
- 58. Synthetic and semi-synthetic antioxidants in medicine and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12321848/]
- 59. Navigating the complexity of lipid oxidation and antioxidation [https://www.sciencedirect.com/science/article/pii/S016378272400050X]
- 60. Antioxidants and Their Physiological Role in Free Radical ... [https://link.springer.com/rwe/10.1007/978-981-96-8622-3_12]
- 61. Polyphenols: A Comprehensive Review of their Nutritional ... [https://www.sciencedirect.com/org/science/article/pii/S1874070721000184]
- 62. Clean-label alternatives for food preservation: An emerging ... [https://www.sciencedirect.com/science/article/pii/S2405844024118464]
- 63. Recent Advances, Challenges, and Functional ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11851614/]
- 64. Antimicrobial Potential of Polyphenols: Mechanisms ... [https://www.mdpi.com/2076-3921/14/2/200]
- 65. Antioxidant, Polyphenol, Physical, and Sensory Changes ... [https://pubmed.ncbi.nlm.nih.gov/40218990/]
- 66. Polyphenol Market Size & Outlook, 2025-2033 [https://straitsresearch.com/report/polyphenol-market]
- 67. Natural polyphenols for sustainable active, smart, and ... [https://www.frontiersin.org/journals/food-science-and-technology/articles/10.3389/frfst.2025.1604816/full]
- 68. Latest Advances in Green Extraction of Polyphenols from ... [https://www.mdpi.com/1420-3049/30/1/55]
- 69. Natural Food Antioxidants Market Set to Grow at 6.4% ... [https://www.openpr.com/news/4273992/natural-food-antioxidants-market-set-to-grow-at-6-4-cagr-through]
- 70. What Is Reduced Oxygen Packaging? [https://westairgases.com/blog/reduced-oxygen-packaging/]
- 71. Oxidation Status and Antioxidant Activity of Analogue Meat ... [https://www.mdpi.com/2076-3417/14/15/6713?]
- 72. Effects of modified atmosphere packaging, vacuum ... [https://www.sciencedirect.com/science/article/pii/S2214289425001267]
- 73. Oxygen Scavenger Innovation for Packaging 2025 Report [https://www.expertmarketresearch.com/technology-reports/oxygen-scavenger-packaging-innovation]
- 74. Oxygen Barrier Films And Coatings For Dry Food Market [https://www.futuremarketinsights.com/reports/oxygen-barrier-films-and-coatings-for-dry-food-market]
- 75. What Is High Barrier Packaging? Benefits & Trends for 2025 [https://tipa-corp.com/blog/what-is-high-barrier-packaging-and-why-does-it-matter-in-2025/]
- 76. Oxygen Barrier Films & Coatings for Dry Food Market Outlook [https://www.gminsights.com/industry-analysis/oxygen-barrier-films-and-coatings-for-dry-food-market]
- 77. Enhancing shelf life of bison meat using CO2/N2 modified ... [https://www.sciencedirect.com/science/article/abs/pii/S0309174025000415]
- 78. Packaging made of board can replace plastic but demands ... [https://nofima.com/results/packaging-made-of-board-can-replace-plastic-but-demands-smarter-coatings-2/]
- 79. Balancing Temperature and Humidity Control in Storage ... [https://www.mdpi.com/2071-1050/17/16/7477]
- 80. Shade of Innovative Food Processing Techniques: Potential Inducing Factors of Lipid Oxidation - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10745320/]
- 81. Underlying mechanisms of synergistic antioxidant ... [https://www.sciencedirect.com/science/article/abs/pii/S0924224423000547]
- 82. Synergistic effects produced by certain antioxidants in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12221894/]
- 83. Synergistic Effects of Antioxidant Blends: A Comparative ... [https://www.mdpi.com/2076-3921/14/8/981]
- 84. Antioxidants: a comprehensive review - PMC - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC12085410/]
- 85. Comparison of the Oxidative Stability and Antioxidant ... [https://www.mdpi.com/2076-3921/8/10/470]
- 86. 4-Hydroxy-2-nonenal in food products: A review of the ... [https://www.sciencedirect.com/science/article/abs/pii/S0924224419306399]
- 87. Oxysterols — how much do we know about food ... [https://www.sciencedirect.com/science/article/abs/pii/S2214799321001132]
- 88. Development, validation and application of an SFC-ESI ... [https://www.sciencedirect.com/science/article/abs/pii/S0308814625040476]
- 89. An Improved GC-MS Method for Malondialdehyde (MDA) ... [https://www.mdpi.com/2304-8158/11/9/1176]
- 90. Measurement of malondialdehyde by high performance ... [https://link.springer.com/article/10.1007/BF02535752]
- 91. 4-Hydroxy-nonenal—A Bioactive Lipid Peroxidation Product [https://pmc.ncbi.nlm.nih.gov/articles/PMC4693237/]
- 92. Aroma compounds in food: Analysis, characterization and ... [https://www.sciencedirect.com/science/article/pii/S2772275925000073]
- 93. Trial Analysis of the Relationship between Taste and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9738716/]
- 94. Effects of beverages and salads on lipid oxidation ... [https://www.nature.com/articles/s41598-025-98977-y]
- 95. Potential effects of lipid lowering and antioxidant activity ... [https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2025.1560462/full]
- 96. Combining In Vitro, In Vivo, and In Silico Approaches to ... [https://pubmed.ncbi.nlm.nih.gov/39852379/]
- 97. The Interplay Between Oxidative Stress and Lipid ... [https://www.mdpi.com/1422-0067/26/17/8544]
- 98. Evolution of oxidation markers and rancid off-flavors in ... [https://link.springer.com/article/10.1007/s10068-025-01999-5]
- 99. Recent Developments and Applications of Food-Based ... [https://www.mdpi.com/2504-5377/9/5/61]
- 100. High internal phase Pickering emulsion by ultrasound ... [https://www.frontiersin.org/journals/sustainable-food-systems/articles/10.3389/fsufs.2025.1529154/full]
- 101. Fat reduction and lipid profile optimization in Spanish ... [https://link.springer.com/article/10.1007/s00217-025-04682-5]
- 102. Linseed, Walnut, and Algal Oil Emulsion Gels as Fat ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12346848/]
- 103. Lipid oxidation in pathophysiology of atherosclerosis [https://pmc.ncbi.nlm.nih.gov/articles/PMC9434419/]
- 104. Inflammation in atherosclerosis: pathophysiology and ... [https://www.nature.com/articles/s41419-024-07166-8]
- 105. Lipid Oxidation Products on Inflammation-Mediated ... [https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2021.717740/full]
- 106. Atherosclerosis: from lipid-lowering and anti-inflammatory ... [https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1485801/full]
- 107. Therapeutic approaches to drug targets in atherosclerosis [https://pmc.ncbi.nlm.nih.gov/articles/PMC4099571/]
- 108. Inflammation and atherosclerosis: signaling pathways and ... [https://www.nature.com/articles/s41392-022-00955-7]
- 109. Results From the OCEAN(a)-DOSE Trial [https://pubmed.ncbi.nlm.nih.gov/39937508/]
- 110. Olpasiran, Oxidized Phospholipids, and Systemic ... [https://scholars.mssm.edu/en/publications/olpasiran-oxidized-phospholipids-and-systemic-inflammatory-biomar/]
- 111. Olpasiran Cuts Lp(a) but Not Inflammatory Markers [https://www.tctmd.com/news/olpasiran-cuts-lpa-not-inflammatory-markers-oceana-dose]