Dietary Triglycerides and Phospholipids: Molecular Structures, Metabolic Pathways, and Clinical Implications
This article provides a comprehensive analysis of the distinct chemical structures of dietary triglycerides and phospholipids and their profound influence on metabolism, health, and disease.
Dietary Triglycerides and Phospholipids: Molecular Structures, Metabolic Pathways, and Clinical Implications
Abstract
This article provides a comprehensive analysis of the distinct chemical structures of dietary triglycerides and phospholipids and their profound influence on metabolism, health, and disease. Tailored for researchers, scientists, and drug development professionals, it synthesizes foundational biochemistry with contemporary research. The scope spans from structural biology and analytical methodologies to the optimization of lipid-based formulations and a comparative review of their roles in cardiometabolic health, neurological function, and pharmaceutical applications. The content is structured to bridge basic science with translational research, highlighting opportunities for therapeutic innovation.
Deconstructing Lipid Architectures: From Glycerol Backbones to Functional Assemblies
The glycerol backbone, a simple three-carbon polyol, serves as a fundamental architectural platform for an immense diversity of biological lipids. This universal core, when esterified with various fatty acid chains and head groups, gives rise to both triglycerides—the primary energy storage molecules in diets—and phospholipids—the essential structural components of cellular membranes. The specific chemical modifications at each carbon position on the glycerol scaffold dictate the ultimate physicochemical properties, metabolic fate, and biological functions of the resulting lipid species. This whitepaper delves into the structural principles governing this diversity, summarizes quantitative data on lipid composition, outlines key experimental methodologies for their study, and visualizes critical metabolic pathways, providing a technical resource for researchers and drug development professionals.
The glycerol backbone, chemically defined as propane-1,2,3-triol, is the central structural component of most lipids in living organisms [1] [2]. Its significance stems from a simple yet versatile architecture: a three-carbon chain where each carbon bears a hydroxyl group, enabling it to form ester linkages with a wide array of fatty acids and other functional groups [3]. This capacity for regioselective substitution transforms this simple molecule into a platform for an astonishing array of complex lipids with vastly different biological roles.
In biological systems, the glycerol backbone is found in two primary enantiomeric forms, which have profound implications for membrane biochemistry and evolution. Eukaryotes and bacteria utilize sn-glycerol-3-phosphate, whereas archaea utilize sn-glycerol-1-phosphate as the backbone for their membrane lipids [1]. This review will focus on the roles of the glycerol backbone in the context of dietary triglycerides and physiological phospholipids, exploring how minimal alterations to its substituents create lipids tailored for functions ranging from long-term energy storage to the formation of dynamic cellular membranes and sophisticated signaling cascades.
Structural Classes of Glycerolipids
The glycerol backbone gives rise to several major classes of lipids, primarily classified based on the nature of the groups attached to the three carbon positions. The table below summarizes the core structures and primary functions of the main glycerolipid classes.
Table 1: Major Classes of Glycerolipids and Their Core Characteristics
| Lipid Class | Backbone Substitutions | Core Structure | Primary Functions |
|---|---|---|---|
| Triacylglycerols (TAGs) [3] | Three fatty acids | Glycerol + 3 Fatty Acyl Chains | Energy storage, thermal insulation [3] |
| Phosphatidylcholine (PC) [4] | Two fatty acids, Phosphate-Choline | Glycerol + 2 Fatty Acyl Chains + Phosphate + Choline | Major eukaryotic membrane lipid; membrane structure and fluidity [3] [5] |
| Phosphatidylethanolamine (PE) [4] | Two fatty acids, Phosphate-Ethanolamine | Glycerol + 2 Fatty Acyl Chains + Phosphate + Ethanolamine | Inner leaflet of plasma membrane; membrane curvature [3] [5] |
| Phosphatidylserine (PS) [4] | Two fatty acids, Phosphate-Serine | Glycerol + 2 Fatty Acyl Chains + Phosphate + Serine | Apoptosis signaling, cell recognition [3] |
| Phosphatidylinositol (PI) [4] | Two fatty acids, Phosphate-Inositol | Glycerol + 2 Fatty Acyl Chains + Phosphate + Inositol | Precursor for intracellular signaling molecules [3] |
| Phosphatidylglycerol (PG) [4] | Two fatty acids, Phosphate-Glycerol | Glycerol + 2 Fatty Acyl Chains + Phosphate + Glycerol | Plant and bacterial membranes; precursor to Cardiolipin [6] |
| Cardiolipin (CL) [3] | Four fatty acids, Two Phosphates | Two Glycerol Backbones + 4 Fatty Acyl Chains + 2 Phosphates | Inner mitochondrial membrane function, apoptosis regulation [3] |
| Monogalactosyldiacylglycerol (MGDG) [6] | Two fatty acids, Monogalactose | Glycerol + 2 Fatty Acyl Chains + Galactose | Main lipid of thylakoid membranes in photosynthesis [7] [6] |
Triglycerides: Dietary Energy Reservoirs
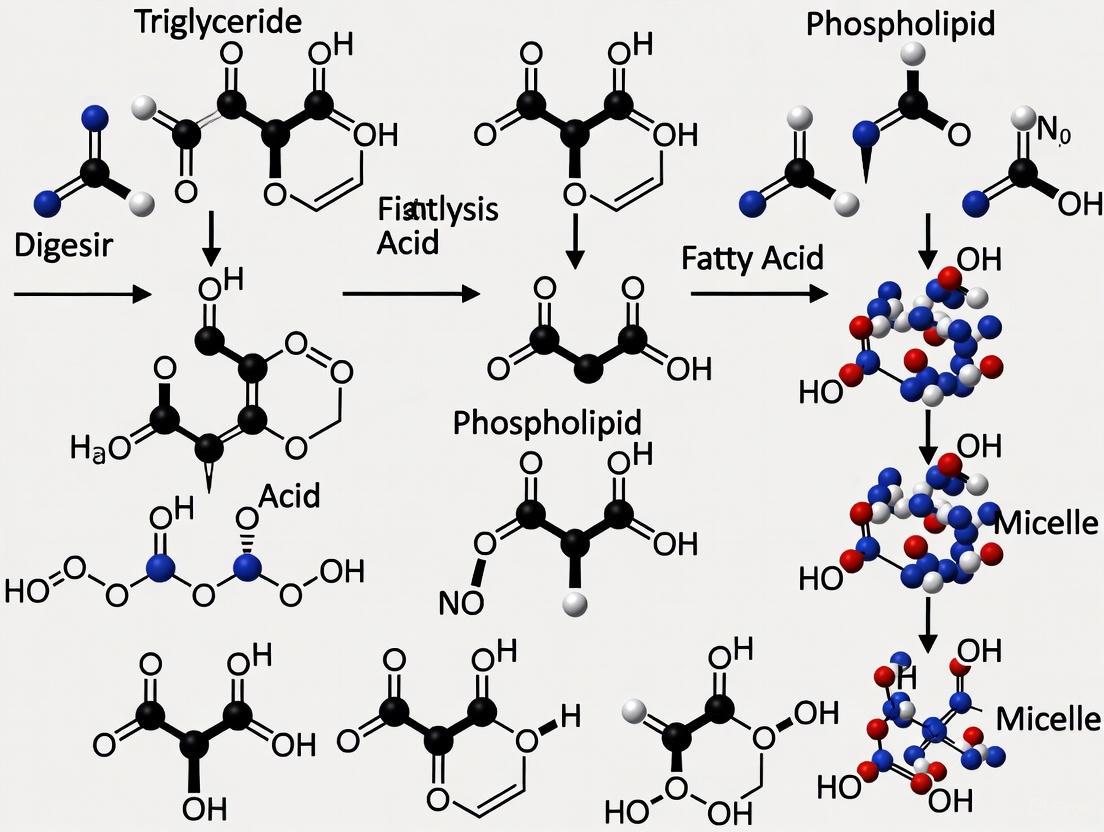
Triacylglycerols (TAGs), or triglycerides, consist of a glycerol backbone esterified with three fatty acid molecules [3]. This structure makes them highly hydrophobic and inert, ideal for compact energy storage in lipid droplets within adipose tissue [3]. The fatty acid composition of dietary TAGs—namely, the chain length, degree of saturation, and precise position (sn-1, sn-2, or sn-3) on the glycerol backbone—directly influences their nutritional value, physical properties (like melting point), and metabolic processing in the body [8]. The hydrolysis of TAGs by lipases releases free fatty acids and glycerol, which can then be utilized for energy production or gluconeogenesis [1].
Phospholipids: Architects of the Cellular Membranes
Glycerophospholipids (or phospholipids) are amphipathic molecules in which the glycerol backbone's sn-1 and sn-2 positions are esterified with fatty acids, and the sn-3 position is linked to a phosphate group. This phosphate group is, in turn, often esterified to a polar head group such as choline, ethanolamine, serine, or inositol [4] [3]. This structure creates a molecule with a hydrophobic tail (the fatty acids) and a hydrophilic head, enabling the spontaneous formation of the lipid bilayer that constitutes all cellular membranes [2]. Phospholipids are not merely structural; they are functional molecules involved in signal transduction, membrane trafficking, and the regulation of membrane-protein interactions [9] [5].
Glycolipids and Specialized Glycerolipids
Glycolipids, such as the monogalactosyldiacylglycerol (MGDG) prevalent in plant chloroplast membranes, feature a sugar moiety attached directly to the glycerol backbone [7] [6]. Other complex glycerolipids include cardiolipin, a unique dimeric phospholipid essential for the function of mitochondrial respiratory complexes [3], and glycero-glycophospholipids like phosphatidylglucoside (PtdGlc), which contain both a phosphate and a sugar in their head group and are involved in cell differentiation and signaling [7].
Quantitative Composition and Physical Properties
The composition of fatty acids attached to the glycerol backbone is highly regulated and varies significantly between different lipid classes, tissues, and organellar membranes. This variation directly impacts membrane physical properties such as fluidity and rigidity.
Table 2: Representative Fatty Acid Compositions of Membrane Glycerophospholipids
| Fatty Acid Notation | Common Name | Typical Role in Membrane Properties | Prevalence in Phospholipids |
|---|---|---|---|
| C16:0 | Palmitic Acid | Saturated; increases membrane rigidity and order | Major component in PC, PE, PG [4] |
| C18:0 | Stearic Acid | Saturated; contributes to membrane packing and stability | Found in PC, PE, PS [4] |
| C18:1 | Oleic Acid | Monounsaturated; introduces kinks, enhances fluidity | Common in most phospholipid classes [4] |
| C18:2 | Linoleic Acid | Polyunsaturated; significantly increases fluidity | Found in various phospholipids, levels can vary with diet [4] |
| C18:3 (α) | α-Linolenic Acid (ALA) | Polyunsaturated; precursor for longer omega-3 fatty acids | Found in various phospholipids, levels can vary with diet [4] |
| C20:4 | Arachidonic Acid (AA) | Polyunsaturated; precursor for eicosanoid signaling molecules | Enriched in certain PI and PS pools for signaling [4] |
Table 3: Glycerolipid Distribution in Cellular Membranes (Mol % of Total Lipids)
| Lipid Class | Representative Cell (Plasma Membrane) | Neuron | Astrocyte | Mitochondrial Membrane (Inner) |
|---|---|---|---|---|
| Phosphatidylcholine (PC) | 45-55% [5] | ~40% [5] | ~40% [5] | ~40% [5] |
| Phosphatidylethanolamine (PE) | 15-25% [5] | ~15% [5] | ~20% [5] | ~35% [5] |
| Phosphatidylinositol (PI) | 10-15% [5] | ~2% [5] | ~5% [5] | <5% |
| Phosphatidylserine (PS) | 5-10% [5] | ~2% [5] | ~8% [5] | <5% |
| Cardiolipin (CL) | 2-5% [5] | <1% [5] | <1% [5] | ~15% [5] |
| Sphingolipids | 5-15% [5] | ~2% [5] | ~10% [5] | Low |
| Sterol Lipids | 10-20% [5] | ~40% [5] | ~25% [5] | Low |
Experimental Methodologies for Analysis
X-Ray Scattering for Nanostructural Analysis
Principle: X-ray scattering is an invaluable tool for investigating the nanostructural properties of crystalline triglycerides and the lamellar structures of phospholipid bilayers. Small-angle X-ray scattering (SAXS) provides information on long-range ordering, such as lamellar repeat distances, while wide-angle X-ray scattering (WAXS) reveals short-range ordering, including polymorphic forms and hydrocarbon chain packing [8].
Detailed Protocol for Triglyceride Polymorphism Analysis:
- Sample Preparation: Pure triglycerides or complex fat systems are melted and then crystallized under controlled temperature and cooling rates to promote the formation of a specific polymorph.
- Data Collection: Simultaneous SAXS and WAXS data are collected, typically using a synchrotron radiation source for high intensity and resolution. The scattering vector range of 0.05 < q < 7 nm⁻¹ is used for SAXS, and 7 < q < 20 nm⁻¹ for WAXS [8].
- Data Analysis:
- Lamellar Stacking: SAXS peaks are analyzed using Bragg's law to determine the long spacing, which corresponds to the distance between crystal lamellae.
- Polymorphic Identification: The short spacings from WAXS patterns are characteristic of the chain packing subcell (e.g., α, β', β) and are used to identify the polymorphic form [8].
- Electron Density Profile (EDP): SAXS data can be used to calculate the EDP, decomposing the lamellar repeat distance into bilayer and monolayer contributions.
- Crystallite Size: The Scherrer equation is applied to the full-width at half maximum (FWHM) of diffraction peaks to estimate the crystallite size and lattice strain [8].
- Chain Tilt and Area: The chain tilt angle within the bilayer and the area per hydrocarbon chain can be estimated by combining information from SAXS and WAXS data [8].
Enzymatic Synthesis of Complex Phospholipids
Principle: Phospholipase D (PLD) catalyzes the transphosphatidylation reaction, enabling the head group exchange of natural phospholipids to synthesize rare or complex phospholipid species in a sustainable and regioselective manner [10].
Detailed Protocol for Synthesis of Hemi-BMPs/BDPs:
- Reaction Setup: A phospholipid substrate (e.g., phosphatidylcholine from soy or egg) is dissolved in an appropriate buffer with a high concentration of an alcohol donor, such as a specific monoacylglycerol (MAG) or diacylglycerol (DAG) regioisomer.
- Enzymatic Catalysis: PLD from Streptomyces sp. is added to the mixture. The enzyme shows broad substrate promiscuity towards both phospholipids and alcohol donors.
- Reaction Monitoring: The reaction is typically carried out for about 2 hours and monitored by thin-layer chromatography (TLC) to track the consumption of the starting material and the formation of the target phospholipid, such as Hemi-bis(monoacylglycero)phosphate (Hemi-BMP) or bis(diacylglycero)phosphate (BDP).
- Product Isolation: The reaction is stopped, and the products are extracted using organic solvents (e.g., chloroform/methanol). The target complex phospholipid is purified using successive column chromatography techniques, yielding a pure product as confirmed by NMR and mass spectrometry [7] [10].
Mass Spectrometry-Based Lipidomics
Principle: Modern mass spectrometry (MS), particularly coupled with liquid chromatography (LC), allows for the comprehensive identification and quantification of thousands of individual lipid species from complex biological extracts. This is crucial for profiling alterations in glycerolipid metabolism in disease states like Amyotrophic Lateral Sclerosis (ALS) [5].
Detailed Workflow:
- Lipid Extraction: Lipids are extracted from tissues, cells, or biofluids using a validated method like Bligh and Dyer, ensuring the recovery of a broad range of glycerolipid classes.
- Chromatographic Separation: The extract is subjected to LC separation, often using reverse-phase columns, to reduce sample complexity and ion suppression before MS analysis.
- Mass Spectrometry Analysis: The eluting lipids are analyzed using high-resolution mass spectrometers. Data-Dependent Acquisition (DDA) or Data-Independent Acquisition (DIA) modes can be used to fragment ions and obtain structural information.
- Data Processing and Integration: Specialized software is used to align peaks, identify lipid species based on their mass-to-charge ratio and fragmentation patterns, and perform relative or absolute quantification. The data is often integrated into resources like the Neurolipid Atlas for cross-study comparison [5].
Pathways and Workflows: A Visual Guide
Glycerophospholipid Biosynthesis Pathway
Diagram Title: Glycerophospholipid Biosynthesis Network
Lipidomics Experimental Workflow
Diagram Title: Lipidomics Analysis Pipeline
The Scientist's Toolkit: Essential Research Reagents
Table 4: Key Reagents and Materials for Glycerolipid Research
| Reagent/Material | Function in Research | Specific Application Example |
|---|---|---|
| Phospholipase D (PLD) | Catalyzes transphosphatidylation for headgroup exchange | Sustainable synthesis of complex phospholipids like Hemi-BMPs from natural PC [10] |
| Synchrotron X-ray Source | Provides high-intensity, tunable X-ray radiation | High-resolution SAXS/WAXS for analyzing triglyceride polymorphic structures and transitions [8] |
| Deuterated Solvents (e.g., CDCl₃) | NMR-active solvents for structural analysis | Determining the structure and stereochemistry of novel glycerolipids like phosphatidylglucoside (PtdGlc) [7] |
| Silica Gel Stationary Phase | Medium for chromatographic separation | Purification of specific glycerolipid classes (e.g., PC, PE) or individual molecular species from complex extracts [7] [4] |
| High-Purity Acyl-CoA Donors | Activated fatty acid donors for enzymatic acylation | Substrates for in vitro studies of glycerol-3-phosphate acyltransferases (GPATs) in phosphatidic acid synthesis [5] |
| Specific Antibodies | Immunodetection of rare lipids | Identification and localization of trace glycero-glycophospholipids like PtdGlc in tissue sections [7] |
The glycerol backbone is a testament to evolutionary parsimony, where a simple molecular scaffold has been leveraged to generate an extensive library of complex lipids through regioselective biochemical decoration. The structural diversity arising from this platform directly underpins the functional dichotomy between triglycerides as energy reservoirs and phospholipids as membrane architects and signaling mediators. A deep understanding of the chemical principles governing this diversity—including fatty acid chain geometry, head group identity, and biosynthetic pathways—is fundamental for research fields ranging from nutritional science and membrane biophysics to drug delivery and the study of neurodegenerative diseases. Continued advancements in analytical techniques like X-ray scattering and lipidomics, coupled with enzymatic synthesis methods, promise to further unravel the complexities of glycerolipid function and their roles in health and disease.
A triglyceride, also known as triacylglycerol (TAG), is a central chemical entity in lipid science, defined as an ester derived from the alcohol glycerol and three fatty acid molecules [11]. This structure serves as the main constituent of body fat in humans, other vertebrates, and vegetable fat [11]. Within the context of dietary lipids and phospholipids research, the triglyceride structure represents the primary form of energy storage and dietary lipid intake, contrasting with the bilayer-forming, structural role of phospholipids [12] [13]. The compositional specificity and structural differences between triglycerides and structural polar lipids, such as the complete "inversion" of fatty acid distribution observed in tissues like pig kidney, point to distinct biosynthetic pathways and functional roles within organisms [13]. A precise understanding of the triglyceride molecule—its isomeric forms, polymorphic crystal behavior, and structure-function relationships—is foundational for research aimed at developing therapeutics for conditions like hypertriglyceridemia, a significant risk factor for cardiovascular and metabolic diseases [14] [15] [16].
Structural Composition and Molecular Geometry
The fundamental architecture of a triglyceride consists of a glycerol backbone serving as a three-carbon hub, with each of its hydroxyl groups esterified to a fatty acid carboxyl group [11] [17]. The three fatty acid substituents can be identical, forming a simple triglyceride (e.g., tristearin), but are most often different, resulting in a mixed triglyceride [11] [12]. The specific positions of these fatty acids on the glycerol molecule are designated using stereospecific numbering (sn) as sn-1, sn-2, and sn-3 [11].
- Glycerol Backbone: A triol (three hydroxyl groups) that forms the structural core of the molecule [18].
- Fatty Acids: Long-chain carboxylic acids with varying lengths (typically 16, 18, or 20 carbon atoms) and degrees of saturation [11] [19]. The chain length and saturation determine the physical and metabolic properties of the triglyceride.
- Ester Bonds: The chemical linkage formed from the reaction between each glycerol hydroxyl group and a fatty acid carboxyl group, releasing a water molecule per esterification [11] [17].
The chain lengths of the fatty acids and their placement on the glycerol backbone are not random. In many natural fats, the distribution is regiospecific. For instance, in most vegetable oils, the saturated palmitic (C16:0) and stearic (C18:0) acid residues are typically attached to positions sn-1 and sn-3, whereas the middle position (sn-2) is usually occupied by an unsaturated fatty acid, such as oleic (C18:1, ω–9) or linoleic (C18:2, ω–6) [11]. This regiochemistry has profound implications for the triglyceride's physical behavior, its digestion by lipases, and its biological effects [12].
Visualizing Triglyceride Structure and Biosynthesis
The following diagram illustrates the esterification reaction that forms a triglyceride and highlights the stereospecific numbering of the glycerol backbone.
Classification and Physical Properties
Triglycerides are classified based on the chemical nature of their constituent fatty acids, which directly dictates their physical state and metabolic fate.
- Saturated Triglycerides: Composed of triglycerides where all fatty acids contain no carbon-carbon double bonds (C=C groups). The carbon chains are "saturated" with hydrogen atoms, allowing them to pack tightly. This results in a higher melting point, making them solids (fats) at room temperature (e.g., stearin from animal tallow) [11] [19].
- Unsaturated Triglycerides: Contain one or more fatty acids with at least one double bond. This introduces "kinks" (cis configuration) in the hydrocarbon chain, preventing tight packing and leading to a lower melting point. They are often liquids (oils) at room temperature (e.g., triolein in olive oil) [11] [19].
- Monounsaturated (MUFA): One double bond in the fatty acid chains.
- Polyunsaturated (PUFA): Two or more double bonds in the fatty acid chains.
The physical properties of triglycerides, such as melting point and density, are heavily influenced by their molecular structure. Saturated fats have higher melting points than unsaturated analogs with the same molecular weight [11]. Furthermore, even a single triglyceride species can exist in multiple crystalline forms known as polymorphs (α, β, and β'), which differ in their melting points and packing geometries [11] [8]. This polymorphic behavior is critical in food science (e.g., chocolate tempering) and pharmaceutical development.
Quantitative Data: Fatty Acid Chain Length and Melting Behavior
The table below summarizes the classification of triglycerides based on fatty acid chain length and provides examples with their typical physical states.
Table 1: Classification of Triglycerides by Fatty Acid Chain Length and Saturation
| Classification | Chain Length (Carbons) | Example | Physical State at Room Temp | Common Sources |
|---|---|---|---|---|
| Long-Chain | 16, 18, 20 | Tristearin (C18:0) | Solid | Animal fats (tallow, lard), cocoa butter [11] |
| Medium-Chain | Shorter than C16 | Triacylglycerols with C8-C12 | Liquid/Semi-solid | Coconut oil, palm kernel oil [11] |
| Saturated | Varies (no C=C bonds) | Tristearin | Solid | Butter, cheese, lard [11] [18] |
| Monounsaturated | Varies (one C=C bond) | Triolein | Liquid | Olive oil, canola oil, peanuts [11] [19] |
| Polyunsaturated | Varies (≥ two C=C bonds) | Linoleic/Linolenic acids | Liquid | Soybean oil, corn oil, fatty fish [11] [19] |
Analytical and Experimental Protocols
Understanding triglyceride structure and function requires robust analytical methods. Enzymatic assays are standard for concentration measurement, while X-ray scattering techniques provide deep insights into crystalline nanostructure.
Standard Enzymatic Assay for Triglyceride Quantification
This protocol is widely used in clinical and research settings to determine triglyceride concentrations in biological samples like serum [15].
Principle: Triglycerides are hydrolyzed to glycerol and free fatty acids. The glycerol is then enzymatically quantified through a series of coupled reactions that produce a colored chromogen, the intensity of which is proportional to the original triglyceride concentration [15].
Detailed Workflow:
Hydrolysis:
Triglyceride + H₂O → Lipoprotein Lipase → Glycerol + 3 Fatty Acids[15]Phosphorylation:
Glycerol + ATP → Glycerol Kinase → Glycerol-3-phosphate[15]Oxidation:
Glycerol-3-phosphate + O₂ → Glycerol-3-phosphate Oxidase → Dihydroxyacetone Phosphate + H₂O₂[15]Chromogen Formation (Color Reaction):
H₂O₂ + 4-Aminoantipyrine (4-AAP) + 4-Chlorophenol → Peroxidase → Quinoneimine Dye (Red) + H₂O[15]
Materials and Reagents [15]:
- Working Reagent: Contains lipoprotein lipase, ATP, glycerol kinase, glycerol-3-phosphate oxidase, peroxidase, 4-AAP/4-aminophenazone, and 4-chlorophenol.
- Triglyceride Standard (200 mg/dL).
- Sample: Serum or heparinized plasma.
- Equipment: Spectrophotometer, test tubes, pipettes.
Procedure [15]:
- Pipette into labeled test tubes (Blank, Standard, Test):
- Blank: 0.01 mL Water + 1 mL Working Reagent
- Standard: 0.01 mL Triglyceride Standard + 1 mL Working Reagent
- Test: 0.01 mL Sample + 1 mL Working Reagent
- Mix and incubate for 10 minutes at room temperature.
- Measure absorbance of Standard (ODS) and Test (ODT) against the Blank at 505 nm.
Calculation [15]: Triglyceride Concentration (mg/dL) = [(ODT – ODB) / (ODS – ODB)] × Concentration of Standard (200 mg/dL)
X-Ray Scattering for Polymorph and Nanostructure Analysis
X-ray scattering is an invaluable tool for characterizing the nanostructural aspects of pure triglycerides and complex fat systems, providing data on polymorphic states, phase transitions, and crystallite size [8].
Key Techniques:
- Small-Angle X-Ray Scattering (SAXS): Reveals information about lamellar stacking and long-range ordering (repeat distances ~0.05 < q < 7 nm⁻¹) [8].
- Wide-Angle X-Ray Scattering (WAXS): Provides details about the polymorphic state and chain packing density within the crystal lattice (q ~7 < 20 nm⁻¹) [8].
Experimental Data Analysis Workflow [8]:
- Data Collection: Perform SAXS/WAXS on triglyceride samples under controlled temperature conditions to monitor crystallization and melting.
- Electron Density Profile (EDP) Calculation: Decomposes the lamellar repeat distance (from SAXS) into bilayer and monolayer contributions.
- Chain Tilt Angle Estimation: Determines the inclination of hydrocarbon chains within the crystal structure.
- Crystallite Size and Strain Analysis: Uses peak broadening (e.g., Scherrer equation) to estimate the size of ordered crystalline domains.
- Area Per Hydrocarbon Chain Calculation: Provides insights into packing geometry and density.
Visualizing the Enzymatic Assay Workflow
The following diagram outlines the sequential reactions in the standard enzymatic assay for triglyceride quantification.
The Scientist's Toolkit: Key Research Reagents and Materials
This section details essential reagents, compounds, and materials used in triglyceride research, from basic analysis to advanced drug development.
Table 2: Essential Reagents and Materials for Triglyceride Research
| Reagent/Material | Function/Description | Research Application |
|---|---|---|
| Lipoprotein Lipase (LPL) | Key enzyme hydrolyzing triglycerides in lipoprotein particles [15]. | Triglyceride clearance studies; enzymatic assay reagent [15]. |
| Olezarsen (IONIS-APOCIII-LRx) | An investigational antisense oligonucleotide targeting apolipoprotein C-III mRNA [16]. | Phase III clinical trial drug for lowering triglycerides in high-risk patients [16]. |
| SEFA-1024 (NorthSea Therapeutics) | An oral, semi-synthetic eicosapentaenoic acid (EPA) derivative [14]. | Phase II investigational drug for severe hypertriglyceridemia (sHTG) [14]. |
| 4-Aminoantipyrine (4-AAP) | Chromogenic substrate that forms a red quinoneimine dye with H₂O₂ [15]. | Colorimetric detection in enzymatic triglyceride quantification assays [15]. |
| GC304 (Genecradle Therapeutics) | A recombinant adeno-associated virus (AAV) vector carrying the LPL gene [14]. | Phase I gene therapy for severe hypertriglyceridemia via long-term LPL expression [14]. |
| Working Reagent (Enzymatic Kit) | Pre-mixed solution containing all necessary enzymes (lipase, GK, GPO), co-factors (ATP), and chromogens [15]. | Standardized high-throughput measurement of serum/plasma triglyceride levels [15]. |
Research and Clinical Implications
The detailed understanding of triglyceride structure directly informs drug discovery and clinical management of dyslipidemias. Elevated triglycerides (hypertriglyceridemia) are an independent risk factor for atherosclerotic cardiovascular disease (ASCVD) and pancreatitis [15] [16]. Modern therapeutic strategies are moving beyond general lipid-lowering to target specific pathways involved in triglyceride metabolism.
A prominent example is Olezarsen, an apolipoprotein C-III (apoC-III) targeting drug. Apolipoprotein C-III inhibits the activity of lipoprotein lipase (LPL), the enzyme responsible for hydrolyzing triglycerides in circulating lipoproteins [16]. By reducing apoC-III production, Olezarsen enhances triglyceride clearance. In the recent ESSENCE-TIMI 73b Phase III trial, monthly subcutaneous Olezarsen injections in patients with moderate hypertriglyceridemia and high cardiovascular risk resulted in an approximate 60% reduction in triglyceride levels at 6 months compared to placebo, with over 80% of patients achieving normal triglyceride levels (<150 mg/dL) [16]. This demonstrates how targeting a specific regulatory protein, rooted in a deep understanding of triglyceride metabolism, can yield potent therapeutic effects.
Other innovative approaches in the pipeline include:
- Gene Therapy: GC304, an AAV vector carrying the beneficial LPLS447X gene variant, aims to provide long-term enzymatic activity to degrade triglycerides [14].
- Multi-Agonists: DR10624 is a first-in-class long-acting tri-agonist targeting FGF21R, GLP-1R, and GCGR, showing promise in reducing body weight and triglycerides in preclinical studies [14].
These advancements underscore the critical role of fundamental structural biology in driving translational research and developing next-generation therapeutics for metabolic diseases.
Phospholipids represent a critically important class of lipids that serve as the fundamental architectural components of all biological membranes. These amphipathic molecules possess a unique molecular structure that enables the formation of lipid bilayers—the primary matrix of cellular membranes that separates intracellular components from the extracellular environment while facilitating selective transport and cellular communication [20] [21]. The defining characteristic of phospholipids is their amphipathic nature, which arises from a molecular structure consisting of a hydrophilic, phosphate-containing head group and two hydrophobic fatty acid tails [21] [22]. This structural duality allows phospholipids to spontaneously organize into complex supramolecular structures in aqueous environments, making them indispensable for cellular life and increasingly valuable in pharmaceutical applications.
Within the broader context of research on the chemical structure of dietary triglycerides and phospholipids, understanding phospholipid structure is paramount. While triglycerides serve primarily as energy storage molecules with three fatty acid chains attached to a glycerol backbone, phospholipids exhibit a more complex structural arrangement with distinct polar and non-polar regions that confer membrane-forming capabilities [23] [21]. This review provides a comprehensive technical examination of phospholipid structure, experimental characterization methodologies, and emerging applications in pharmaceutical research, with particular emphasis on the relationship between molecular structure and biological function.
Molecular Architecture of Phospholipids
Fundamental Structural Components
The molecular architecture of phospholipids consists of four principal components that together create the amphipathic character essential for their biological function. These components include a glycerol backbone that serves as the structural foundation, two hydrophobic fatty acid tails that provide the hydrophobic barrier function, a phosphate group that introduces negative charge and polarity, and a variable alcohol-derived head group that determines the specific chemical identity and properties of the phospholipid [20] [21] [22].
The glycerol backbone forms the central core of the phospholipid molecule, with three carbon atoms typically designated as sn-1, sn-2, and sn-3 according to stereospecific numbering convention. The sn-1 and sn-2 positions are esterified with fatty acid chains, while the sn-3 position is linked to a phosphate group [22]. This arrangement creates the fundamental platform upon which the amphipathic character of the molecule is built. The fatty acid tails attached to the glycerol backbone are long hydrocarbon chains typically consisting of 14-24 carbon atoms that may be either saturated (containing no double bonds) or unsaturated (containing one or more double bonds) [20] [22]. The degree of saturation and chain length significantly influences the packing efficiency and fluidity of the membranes formed by phospholipids, with unsaturated chains introducing kinks that prevent tight packing and increase membrane fluidity.
The phosphate group connected to the sn-3 position of the glycerol backbone consists of a phosphorus atom bonded to four oxygen atoms in a tetrahedral arrangement, with one oxygen atom forming a phosphoester bond with glycerol [21]. This group carries a negative charge under physiological pH conditions, contributing significantly to the hydrophilic nature of the phospholipid head region. The head group is attached to the phosphate group through a phosphoester bond and determines the specific class and chemical properties of the phospholipid [20] [22]. Common naturally occurring head groups include choline (forming phosphatidylcholine), ethanolamine (forming phosphatidylethanolamine), serine (forming phosphatidylserine), inositol (forming phosphatidylinositol), and glycerol (forming phosphatidylglycerol). The chemical diversity of these head groups imparts distinct biophysical properties, charge characteristics, and biological functionalities to different phospholipid classes.
Classification and Structural Variants
Phospholipids are classified based on the nature of their head group, backbone structure, and fatty acid composition. The major classes of glycerophospholipids include phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), phosphatidylglycerol (PG), and phosphatidic acid (PA) [20]. Each class exhibits distinct charge characteristics, hydrogen-bonding capabilities, and membrane properties that influence biological function. For instance, phosphatidylcholine and phosphatidylethanolamine are zwitterionic under physiological conditions, while phosphatidylserine carries a net negative charge and phosphatidylinositol can be phosphorylated at multiple positions to create signaling molecules such as PIP₂ (phosphatidylinositol 4,5-bisphosphate) [20].
The structural notation for phospholipids follows a standardized convention that specifies the head group type and the fatty acid composition. For example, PC(16:0/18:1) denotes a phosphatidylcholine molecule with a palmitic acid (16:0, saturated) at the sn-1 position and an oleic acid (18:1, monounsaturated) at the sn-2 position [22]. This notation provides precise information about the molecular structure that correlates with physical properties such as phase transition temperature, membrane curvature, and lateral organization.
Table 1: Major Phospholipid Classes and Their Structural Characteristics
| Phospholipid Class | Head Group | Charge at pH 7.4 | Abundance in Mammalian Membranes | Key Structural Features |
|---|---|---|---|---|
| Phosphatidylcholine (PC) | Choline | Zwitterionic | 40-50% | Cylindrical shape; promotes bilayer formation |
| Phosphatidylethanolamine (PE) | Ethanolamine | Zwitterionic | 20-25% | Conical shape; promotes hexagonal phases |
| Phosphatidylserine (PS) | Serine | Negative | 5-10% | Localized to inner leaflet; apoptosis marker |
| Phosphatidylinositol (PI) | Inositol | Negative | 5-10% | Signaling precursor; can be phosphorylated |
| Phosphatidylglycerol (PG) | Glycerol | Negative | 1-2% | Mitochondrial membrane component |
| Phosphatidic Acid (PA) | Hydrogen | Negative | <1% | Signaling lipid; small head group |
Sphingophospholipids represent another important category of phospholipids that utilize sphingosine rather than glycerol as their backbone. The most prominent member of this class is sphingomyelin, which contains a phosphocholine head group attached to a ceramide unit [20]. Sphingomyelin is particularly abundant in the myelin sheath of nerve cells and forms lipid rafts—specific membrane microdomains involved in cellular signaling. The structural diversity of phospholipids extends to ether-linked variants such as plasmalogens, which contain a vinyl ether linkage at the sn-1 position and are particularly enriched in neural and cardiac tissues.
Amphipathic Nature and Self-Assembly Behavior
The Amphipathic Design Principle
The amphipathic character of phospholipids represents their most defining structural feature, with clear spatial segregation between hydrophilic and hydrophobic regions. The hydrophilic component encompasses the phosphate group and its associated head group, which exhibit polarity and hydrogen-bonding capacity that favors interactions with aqueous environments [21]. In contrast, the hydrophobic component consists of the fatty acid tails, which are nonpolar and exclusively interact with other hydrophobic moieties through van der Waals interactions [21]. This molecular duality drives the spontaneous self-assembly of phospholipids into complex supramolecular structures when dispersed in aqueous solutions.
The amphipathic nature of phospholipids can be visualized through their molecular representation, which shows distinct spatial regions with different solubility parameters. The hydrophilic head orients toward aqueous phases, while the hydrophobic tails minimize contact with water by associating with other hydrophobic chains. This molecular organization arises from the thermodynamic driving force to maximize favorable interactions (head-water and tail-tail) while minimizing unfavorable ones (tail-water). The resulting reduction in free energy provides the impetus for self-assembly processes that create biologically essential structures [21].
Supramolecular Assemblies and Phase Behavior
Depending on concentration, temperature, and molecular structure, phospholipids can form various lyotropic liquid crystalline phases including micelles, bilayers, and hexagonal phases. The critical packing parameter (CPP), defined as CPP = v/(a₀·l), where v is the hydrocarbon chain volume, a₀ is the optimal head group area, and l is the chain length, predicts the preferred supramolecular arrangement [24]. Phospholipids with relatively large head groups and single-chain tails typically form spherical or cylindrical micelles, while those with two hydrocarbon chains and moderate head group sizes preferentially assemble into bilayers [21].
The lipid bilayer represents the most biologically significant supramolecular structure formed by phospholipids. In this arrangement, two monolayers of phospholipids associate tail-to-tail to form a planar sheet approximately 5 nm thick, with the hydrophilic head groups facing the aqueous environment on both sides and the hydrophobic tails forming a continuous internal hydrocarbon core [21]. This configuration effectively separates two aqueous compartments while providing a two-dimensional fluid matrix for membrane proteins. The bilayer structure forms the fundamental architecture of all cellular membranes, including the plasma membrane and various organellar membranes.
Table 2: Phospholipid Self-Assembly Structures and Their Characteristics
| Assembly Structure | Molecular Shape | Critical Packing Parameter | Typical Phospholipids | Biological Relevance |
|---|---|---|---|---|
| Micelle | Cone-shaped | < 1/3 | Lysophospholipids | Lipid digestion, bile acids |
| Bilayer | Cylindrical | ~1 | Phosphatidylcholine | Cellular membranes |
| Hexagonal II (HII) | Inverted cone | > 1 | Phosphatidylethanolamine | Membrane fusion, protein function |
| Cubic Phase | Complex | ~1 | Mixtures | Membrane organization, protein crystallization |
The phase behavior of phospholipids is strongly influenced by temperature, hydration, and molecular structure. Phospholipids undergo thermotropic phase transitions between ordered gel phases and disordered fluid phases at characteristic temperatures known as transition temperatures (Tₘ) [24]. Below Tₘ, the hydrocarbon chains exist in an extended, all-trans conformation and exhibit limited lateral mobility. Above Tₘ, the chains contain gauche conformers that introduce kinks, increasing free volume and enabling lateral diffusion of membrane components. This phase behavior is critically important for membrane function, as biological membranes must maintain fluidity for proper functionality while providing sufficient structural integrity.
Experimental Characterization of Phospholipid Structure
Physicochemical Analysis Techniques
The structural characterization of phospholipids employs a diverse array of biophysical techniques that provide complementary information about molecular organization, phase behavior, and dynamic properties. Differential scanning calorimetry (DSC) measures the heat capacity changes associated with phase transitions, providing quantitative data on transition temperatures, enthalpies, and cooperativity [24]. Isothermal titration calorimetry (ITC) characterizes the thermodynamics of phospholipid interactions with other molecules, including drugs, proteins, and membrane-active compounds.
X-ray scattering (XRD) techniques, including small-angle X-ray scattering (SAXS) and wide-angle X-ray scattering (WAXS), provide detailed structural information about phospholipid assemblies [8] [24]. SAXS measures long-range organization such as lamellar repeat distances in multilamellar vesicles, while WAXS characterizes short-range molecular packing including chain-chain distances and tilt angles. Fourier-transform infrared (FT-IR) spectroscopy probes vibrational modes that are sensitive to conformational order, hydration status, and inter-molecular interactions, particularly in the hydrocarbon chain region [24].
Nuclear magnetic resonance (NMR) spectroscopy, especially ³¹P-NMR and ²H-NMR, provides insights into head group orientation and chain dynamics, respectively [20] [24]. ³¹P-NMR is particularly valuable for characterizing phospholipid head group conformation and phase identification, while ²H-NMR using deuterated chains reveals order parameters that quantify the degree of orientational constraint along the fatty acid chains. Fluorescence spectroscopy utilizing environment-sensitive fluorophores provides information about membrane fluidity, lateral organization, and phase separation in phospholipid membranes [24].
Analytical Methodologies for Structural Elucidation
The comprehensive structural analysis of phospholipid mixtures requires sophisticated analytical separation and detection methods. High-performance liquid chromatography (HPLC) coupled with evaporative light scattering detection (ELSD) enables separation and relative quantification of different phospholipid classes based on their polar head groups [20]. Mass spectrometry, particularly electrospray ionization mass spectrometry (ESI-MS) and matrix-assisted laser desorption/ionization (MALDI-MS), provides detailed information about molecular mass, fatty acyl composition, and regiospecific distribution [22].
Mass spectrometric analysis of phospholipids involves multiple stages, beginning with lipid extraction using organic solvents such as chloroform-methanol mixtures. The extracted lipids are then separated by normal-phase HPLC according to head group polarity or by reversed-phase HPLC based on fatty acyl chain characteristics [22]. Tandem mass spectrometry (MS/MS) with collision-induced dissociation generates characteristic fragment patterns that enable structural identification, including determination of fatty acyl regiospecificity (sn-1 versus sn-2 position) [22]. This analytical approach has been instrumental in establishing lipidomics as a comprehensive methodology for quantifying and characterizing complete phospholipid profiles in biological systems.
Diagram 1: Experimental workflow for comprehensive phospholipid analysis showing the integration of multiple analytical techniques for structural characterization.
Research Reagent Solutions for Phospholipid Studies
Table 3: Essential Research Reagents for Phospholipid Structural Analysis
| Research Reagent | Composition/Type | Experimental Function | Key Applications |
|---|---|---|---|
| Synthetic Phospholipids | DPPC, DOPC, DSPC, POPC | Defined model membranes | Phase behavior studies, membrane protein research |
| Natural Phospholipid Extracts | Soy PC, Egg PC, Liver PI | Biologically relevant mixtures | Membrane biophysics, lipidomics |
| Fluorescent Probes | DPH, NBD-PE, Laurdan | Membrane environment sensors | Fluidity measurements, lipid domain visualization |
| Deuterated Lipids | DMPC-d₅₄, DPPC-d₆₂ | NMR spectroscopy standards | Molecular dynamics, order parameter determination |
| Spin-Labeled Lipids | DOXYL, TEMPO derivatives | EPR spectroscopy | Membrane dynamics, oxygen permeability |
| Lipid Standards | Odd-chain, deuterated | Mass spectrometry | Quantitative lipidomics, identification |
Structural Determinants of Membrane Organization
Lipid Bilayer Formation and Properties
The spontaneous formation of phospholipid bilayers represents the structural foundation of biological membranes. When phospholipids are dispersed in aqueous solution, they spontaneously self-assemble into closed bilayered vesicles known as liposomes [21]. These structures typically range from 50 nm to several micrometers in diameter and encapsulate an aqueous interior, providing a model system for studying membrane properties and functions. The driving force for bilayer formation is the hydrophobic effect, which promotes the sequestration of hydrocarbon chains from water while maintaining favorable interactions between hydrophilic head groups and the aqueous environment [21].
The fluid mosaic model describes the biological membrane as a two-dimensional fluid matrix in which phospholipids can diffuse laterally while proteins and other components are embedded [20]. This model emphasizes the dynamic nature of membranes, with phospholipid molecules exhibiting rapid lateral diffusion (approximately 10⁻⁸ cm²/s) and slower transbilayer movement (flip-flop). Membrane fluidity is modulated by several structural factors including fatty acyl chain length, degree of unsaturation, cholesterol content, and temperature. Shorter chains and cis-unsaturated bonds increase fluidity by reducing packing efficiency, while longer saturated chains and cholesterol decrease fluidity [21].
Asymmetric Distribution and Lateral Organization
Biological membranes exhibit asymmetric distribution of phospholipid classes between the inner and outer leaflets. In eukaryotic plasma membranes, phosphatidylcholine and sphingomyelin are predominantly located in the outer leaflet, while phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol are concentrated in the inner leaflet [20]. This asymmetry is generated and maintained by ATP-dependent lipid transporters such as flippases, floppases, and scramblases. Phospholipid asymmetry has functional consequences for membrane properties, protein recruitment, and cellular signaling, with the exposure of phosphatidylserine in the outer leaflet serving as an important signal for apoptosis.
In addition to transverse asymmetry, biological membranes exhibit lateral heterogeneity with the formation of specialized microdomains known as lipid rafts. These nanoscale domains are enriched in sphingolipids, cholesterol, and specific membrane proteins, and function as organizing centers for cellular signaling [20]. The structural basis for raft formation lies in the preferential packing between saturated sphingolipids and cholesterol, which creates liquid-ordered phases that coexist with the more disordered bulk membrane environment. This lateral organization enables the spatial segregation of signaling components and contributes to the regulation of cellular processes.
Advanced Structural Applications in Pharmaceutical Research
Phospholipids in Drug Delivery Systems
The amphipathic nature and self-assembly properties of phospholipids make them invaluable excipients in pharmaceutical formulations, particularly for advanced drug delivery systems. Liposomes—spherical vesicles consisting of one or more phospholipid bilayers—represent the most extensively developed phospholipid-based delivery platform [20] [25]. These nanostructures can encapsulate hydrophilic drugs within their aqueous interior or incorporate hydrophobic drugs within the lipid bilayer, providing protection from degradation, modifying biodistribution, and enabling controlled release kinetics.
The structural properties of component phospholipids determine critical characteristics of liposomal formulations including size, surface charge, membrane fluidity, and stability. Saturated phospholipids with high phase transition temperatures (e.g., DSPC) form rigid, less permeable bilayers that extend drug retention, while unsaturated phospholipids (e.g., DOPC) create more fluid membranes that enhance release rates [20]. Surface modification with polyethylene glycol (PEG)-conjugated phospholipids creates steric stabilization that reduces opsonization and extends circulation half-life—a key advancement known as PEGylation [25].
Recent innovations in phospholipid-based delivery systems include lipid nanoparticles (LNPs) for nucleic acid delivery, ethosomal systems for enhanced transdermal penetration, and targeted liposomes functionalized with ligands for specific cell types [20] [25]. The successful deployment of LNPs for mRNA COVID-19 vaccines highlights the pharmaceutical importance of phospholipid-based delivery systems, where ionizable phospholipids form complex structures that protect nucleic acid payloads and facilitate cellular uptake [25].
Structural Considerations in Formulation Development
The rational design of phospholipid-based drug delivery systems requires comprehensive understanding of structure-function relationships. Key structural parameters include head group chemistry, fatty acyl chain length and saturation, and regiospecific distribution of fatty acids. For instance, the molecular shape of phospholipids—determined by the relative cross-sectional areas of the head group and acyl chains—influences membrane curvature and fusogenicity [26]. Cone-shaped lipids such as phosphatidylethanolamine promote negative curvature and facilitate membrane fusion, while inverted cone-shaped lysophospholipids induce positive curvature.
The positional distribution of fatty acids on the glycerol backbone significantly impacts biological activity and metabolic fate. Phospholipids with docosahexaenoic acid (DHA) at the sn-2 position adopt a hairpin conformation that facilitates enzymatic recognition and membrane integration, while sn-1 DHA creates U-shaped configurations that profoundly influence membrane fluidity and protein interactions [26]. These structural nuances determine the pharmacological performance of phospholipid-based formulations including their stability, biodistribution, cellular uptake, and intracellular trafficking.
Diagram 2: Structure-function relationships in phospholipid-based drug delivery systems showing how molecular features influence pharmacological performance.
Emerging Research and Future Perspectives
Current Frontiers in Phospholipid Research
Recent advances in phospholipid research have revealed increasingly sophisticated structure-function relationships with important implications for pharmaceutical science. The 8th International Symposium on Phospholipids in Pharmaceutical Research (2024) highlighted several emerging areas including anisotropic lipid nanoparticles, tetraether lipids for enhanced stability, and multifunctional lipopeptides [25]. These developments leverage the unique structural properties of phospholipids to create increasingly sophisticated delivery platforms with enhanced targeting capabilities and improved pharmacokinetic profiles.
Structural phospholipidomics has emerged as a powerful approach for comprehensively characterizing phospholipid molecular species and their biological roles [26]. Advanced mass spectrometry techniques now enable detailed analysis of phospholipid regioisomers and stereoisomers, revealing previously unappreciated structural diversity. The position-specific effects of polyunsaturated fatty acids such as DHA are particularly significant, with sn-1 versus sn-2 localization dictating membrane biophysical properties and metabolic processing [26]. These insights are driving the development of structurally optimized phospholipids with tailored properties for specific therapeutic applications.
Future Directions and Applications
The evolving understanding of phospholipid structure continues to enable innovative applications in pharmaceutical technology and medicine. Current research directions include the design of stimuli-responsive phospholipids that undergo structural changes in response to specific triggers such as pH, enzymes, or light, enabling spatially and temporally controlled drug release [25]. Additionally, the integration of phospholipids with biomaterials and tissue engineering scaffolds creates biomimetic interfaces that modulate cellular responses and promote regeneration.
The development of synthetic phospholipids with non-natural head groups or tailored acyl chains represents another promising frontier [20]. These designer phospholipids can be engineered to exhibit specific properties such as enhanced stability, reduced immunogenicity, or selective enzymatic cleavage. Furthermore, the convergence of phospholipid research with gene therapy and RNA medicine is creating new opportunities for advanced delivery systems, as evidenced by the critical role of ionizable phospholipids in mRNA-LNP formulations [25]. As structural characterization techniques continue to advance, particularly with improvements in cryo-electron microscopy and molecular dynamics simulations, our understanding of phospholipid structure and function will continue to deepen, enabling increasingly sophisticated pharmaceutical applications.
The amphipathic structure of phospholipids, characterized by a phosphate-containing head group and hydrophobic fatty acid tails, represents a remarkable example of molecular design that enables fundamental biological processes and advanced pharmaceutical applications. The structural principles governing phospholipid self-assembly, membrane organization, and molecular interactions provide the foundation for understanding their diverse roles in biological systems and exploiting their properties for therapeutic benefit. Ongoing research continues to reveal new dimensions of phospholipid structural complexity, from position-specific effects of fatty acids to the sophisticated behavior of mixed lipid systems. These advances underscore the enduring importance of phospholipid structure as a rich area of scientific inquiry with significant implications for drug development and human health.
Lipids are a diverse group of hydrophobic or amphiphilic molecules that serve crucial structural and metabolic roles in biological systems. The fundamental classes of lipids include triglycerides, phospholipids, sterols, and waxes [27] [28]. While triglycerides, composed of a glycerol backbone esterified with three fatty acids, constitute more than 95% of dietary lipids and serve primarily as energy storage molecules, phospholipids represent a structurally and functionally distinct class [27] [19]. Phospholipids are amphiphilic molecules, featuring a glycerol backbone attached to two hydrophobic fatty acid "tails" and a hydrophilic phosphate-containing "head" group [27] [28]. This unique structure enables them to form the fundamental architectural matrix of all biological membranes and act as critical signaling molecules [27] [29] [30]. This whitepaper provides an in-depth technical examination of the four major classes of dietary phospholipids—phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and sphingomyelin—framed within the broader context of chemical structure-function relationships in lipid research.
Structural Foundations: Glycerophospholipids vs. Sphingophospholipids
Dietary phospholipids are categorized based on their backbone structure into glycerophospholipids (phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine) and sphingophospholipids (sphingomyelin).
Glycerophospholipids share a common structural blueprint: a glycerol backbone, where the sn-1 and sn-2 positions are esterified to fatty acids of varying chain lengths and saturation, and the sn-3 position is linked to a phosphate group. The identity of the phospholipid is determined by the specific alcohol moiety (e.g., choline, ethanolamine, serine) attached to this phosphate [27] [29] [30]. The fatty acid composition is highly variable, influencing the molecule's physical properties, such as membrane fluidity and melting point [19].
Sphingomyelin is distinct as a sphingophospholipid. Its backbone is sphingosine, an amino alcohol, rather than glycerol. A fatty acid is attached via an amide bond to the sphingosine, forming a ceramide. The primary hydroxyl group of ceramide is then esterified to phosphocholine, giving it a head group identical to phosphatidylcholine [31].
The following diagram illustrates this structural classification and biosynthesis pathway.
Detailed Analysis of Major Phospholipid Classes
Phosphatidylcholine (PC)
Chemical Structure: Phosphatidylcholine consists of a glycerol backbone esterified to two fatty acids and a phosphate group linked to a choline molecule [29]. It is a zwitterionic phospholipid at physiological pH due to the positive quaternary ammonium on choline and the negative phosphate group.
Biosynthesis: The primary pathway for PC synthesis in eukaryotes is the Kennedy pathway, which involves the condensation of diacylglycerol (DAG) with CDP-choline, catalyzed by diacylglycerol cholinephosphotransferase [29]. An alternative pathway in the liver involves the methylation of phosphatidylethanolamine using S-adenosyl methionine (SAM) as the methyl donor [29].
Dietary Sources and Commercial Production: PC is a major component of the lecithin group of substances. Commercial PC is often derived from soybean and egg yolk [29]. As a food emulsifier, it is commonly labeled as "lecithin."
Biological Functions:
- Membrane Integrity: PC is a fundamental building block of all biological membranes, predominantly located in the outer leaflet of the plasma membrane [29].
- Surfactant Function: Dipalmitoylphosphatidylcholine is a critical component of pulmonary surfactant, essential for reducing surface tension in the lungs [29].
- Signal Transduction: PC serves as a substrate for phospholipases, generating lipid second messengers, and is involved in membrane-mediated cell signaling [29].
Phosphatidylethanolamine (PE)
Chemical Structure: PE shares the glycerol backbone with two fatty acids but is esterified to an ethanolamine head group [30]. Its smaller head group creates a molecular cone shape, promoting membrane curvature.
Biosynthesis: PE can be synthesized via the CDP-ethanolamine pathway or by the decarboxylation of phosphatidylserine by phosphatidylserine decarboxylase in the mitochondrial membrane [30].
Dietary Sources: PE is abundant in nervous tissue, soy lecithin, and egg yolk [30].
Biological Functions:
- Membrane Curvature and Fusion: PE's molecular shape is crucial for facilitating membrane fusion and fission events, such as those in cell division and vesicle trafficking [30].
- Cellular Process Support: In bacteria, the principal phospholipid is PE, where it supports the proper folding and function of membrane transport proteins like lactose permease [30].
- Precursor Role: PE is a precursor for the synthesis of phosphatidylcholine (via methylation) and the endocannabinoid anandamide [30].
Phosphatidylserine (PS)
Chemical Structure: PS features a serine amino acid linked to the phosphate group on the glycerol backbone [32]. This gives it a net negative charge at physiological pH.
Biosynthesis: In mammals, PS is synthesized from pre-existing PC or PE via a Ca²⁺-dependent head-group exchange reaction catalyzed by phosphatidylserine synthase 1 or 2 in the endoplasmic reticulum [32].
Dietary Sources: PS is found in meat, fish, and offal. Soy lecithin is a notable plant source, containing about 3% PS of total phospholipids [32].
Biological Functions:
- Apoptotic Signal: A critical function of PS is its role in apoptosis. In healthy cells, PS is almost exclusively confined to the inner leaflet of the plasma membrane. During programmed cell death, PS rapidly translocates to the outer leaflet, acting as an "eat-me" signal for phagocytic cells to clear the dying cell [32].
- Signaling Cofactor: PS located in the cytoplasmic leaflet of the plasma membrane serves as a cofactor for several key signaling proteins, including Akt and Protein Kinase C (PKC), thereby stimulating processes like neuronal survival and growth [32].
Sphingomyelin (SM)
Chemical Structure: SM is built on a ceramide backbone (sphingosine plus a fatty acid) with a phosphocholine head group [31]. Its fatty acids tend to be longer and more saturated than those of glycerophospholipids.
Biosynthesis: SM is synthesized in the Golgi apparatus by the transfer of a phosphocholine group from PC to a ceramide, a reaction catalyzed by sphingomyelin synthase, which also produces diacylglycerol as a byproduct [31].
Dietary Sources: SM is found in animal cell membranes, with particularly high concentrations in the myelin sheath surrounding nerve cells, milk, and egg yolks [31].
Biological Functions:
- Myelin Formation: SM is a major constituent of the myelin sheath, providing insulation and facilitating rapid nerve conduction [31].
- Lipid Raft Formation: Due to its high transition temperature and strong interactions with cholesterol, SM is a key component of lipid rafts—ordered membrane microdomains involved in signal transduction and membrane trafficking [31].
- Signal Transduction: The hydrolysis of SM by sphingomyelinases generates ceramide, a potent lipid second messenger deeply involved in stress response, apoptosis, and cell senescence [31].
Table 1: Comparative Summary of Major Dietary Phospholipid Classes
| Feature | Phosphatidylcholine (PC) | Phosphatidylethanolamine (PE) | Phosphatidylserine (PS) | Sphingomyelin (SM) |
|---|---|---|---|---|
| Backbone Structure | Glycerol | Glycerol | Glycerol | Sphingosine (Ceramide) |
| Polar Head Group | Choline | Ethanolamine | Serine | Phosphocholine |
| Net Charge (pH 7) | Zwitterionic | Zwitterionic | Negative (-1) | Zwitterionic |
| Primary Location in Membrane | Outer & Inner Leaflet (more in outer) | Inner Leaflet (mainly) | Inner Leaflet (exclusively in viable cells) | Outer Leaflet (primarily) |
| Key Biological Functions | Membrane structure, pulmonary surfactant, signaling precursor | Membrane fusion, curvature, chaperone for membrane proteins | Apoptotic signaling, cofactor for PKC/Akt signaling | Myelin formation, lipid rafts, source of ceramide |
| Rich Dietary Sources | Egg yolk, soy lecithin, meat | Nervous tissue, soy lecithin, eggs | Offal (liver, kidney), mackerel, soy lecithin | Animal meats, milk, egg yolk, myelin-rich tissues |
Experimental Protocols in Phospholipid Research
Lipidomics Workflow for Analyzing Dietary Phospholipid Effects
Modern phospholipid research relies heavily on lipidomics to comprehensively analyze lipid compositions and their changes in response to dietary interventions. The following diagram outlines a standard lipidomics workflow based on a cited dietary intervention study [33].
Detailed Protocol Description:
Dietary Intervention Design: A controlled feeding trial is implemented. For example, the DIVAS trial was a 16-week randomized controlled trial (RCT) where an isoenergetic diet high in saturated fatty acids (SFA; 17% total energy) was compared to a diet where 8% of SFA was replaced with unsaturated fatty acids (UFA; primarily from plants) [33]. This design isolates the effect of dietary fat quality on the lipidome.
Sample Collection and Lipid Extraction: Blood samples are collected from fasting participants before and after the intervention. Plasma or serum is separated. Lipids are then extracted using methods like liquid-liquid extraction (e.g., Folch or Bligh & Dyer methods) to isolate the total lipid fraction from proteins and other polar contaminants [33].
Lipidomics Profiling via Mass Spectrometry: The extracted lipids are analyzed using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). This platform can separate and quantify hundreds to thousands of individual lipid molecular species, including the major phospholipid classes (PC, PE, PS, SM) and their constituent fatty acids. The DIVAS trial profiled 987 molecular lipid species [33].
Data Processing and Statistical Analysis: Raw MS data are processed for peak identification, alignment, and absolute or relative quantification. Statistical analysis identifies lipids significantly altered by the intervention. In the DIVAS trial, 45 class-specific fatty acid concentrations were significantly changed after false discovery rate (FDR) correction. These changes are often summarized into a Multi-Lipid Score (MLS) to capture the overall lipidomic response to the dietary intervention [33].
Health Outcome Correlation: The derived MLS or individual lipid changes are then tested for association with health outcomes, such as incident cardiovascular disease (CVD) or type 2 diabetes (T2D), in large prospective cohorts to validate their biological and clinical relevance [33].
Analysis of Phospholipid-Derived Flavor Compounds
Objective: To investigate how dietary phospholipids influence the volatile flavor compound profiles in muscle tissue, as demonstrated in abalone [34].
Methodology:
- Feeding Trial: Conduct a long-term feeding trial (e.g., 106 days) with graded levels of dietary phospholipids (e.g., 0.10% to 2.48%) [34].
- Flavor Compound Analysis: Analyze muscle tissue using Gas Chromatograph-Ion Mobility Spectrometry (GC-IMS). This technique separates and identifies volatile organic compounds based on their retention time and drift time, creating a fingerprint of the flavor profile [34].
- Lipidomics Analysis: Perform comprehensive lipidomic analysis on the same tissue to identify changes in lipid composition.
- Data Integration: Use multivariate statistical methods like Partial Least Squares Regression (PLS-R) to correlate specific lipid species (e.g., PC and PE) with the abundance of specific volatile flavor compounds [34].
Key Findings: The study found that dietary phospholipid levels significantly altered the volatile flavor profile, increasing compounds with pleasant aromas at higher inclusion levels. Lipidomics revealed that phosphatidylcholines (PCs) and phosphatidylethanolamines (PEs) were the main differential lipids, and PLS-R analysis confirmed a strong relationship between changes in these phospholipids and the formation of flavor compounds [34].
Table 2: Key Reagent Solutions for Phospholipid Research
| Research Reagent / Material | Function / Application in Protocol |
|---|---|
| Soybean or Egg Yolk Lecithin | A natural, commercially available source of mixed phospholipids (PC, PE, PI, PS) used in dietary intervention studies and as a standard for analysis [29] [30]. |
| Lipid Standards (e.g., dipalmitoyl-PC, palmitoyl-oleoyl-PE) | Isotopically labeled or unlabeled pure chemical standards used for mass spectrometry calibration, peak identification, and quantitative analysis [33]. |
| LC-MS/MS Grade Solvents (e.g., Chloroform, Methanol, Isopropanol) | High-purity solvents used for lipid extraction from biological samples (e.g., plasma, tissue) and for the mobile phase in liquid chromatography to prevent background interference [33]. |
| Solid Phase Extraction (SPE) Cartridges (e.g., Silica, C18) | Used for pre-analytical clean-up and fractionation of complex lipid extracts to isolate specific phospholipid classes before mass spectrometry analysis. |
| Sphingomyelinase Enzyme | A specific type-C phospholipase used experimentally to hydrolyze sphingomyelin to ceramide and phosphocholine, enabling the study of SM's role in signaling pathways like apoptosis [31]. |
| Gas Chromatograph-Ion Mobility Spectrometry (GC-IMS) | An analytical platform used to separate, detect, and identify volatile organic compounds, applied in research linking phospholipid composition to flavor and aroma profiles in foods [34]. |
Health, Disease, and Nutritional Implications
Phospholipids in Metabolic Health and Disease
Lipidomics studies reveal that dietary fat quality significantly influences the plasma phospholipid profile, which is intimately linked to cardiometabolic health. Replacing saturated fats (SFA) with unsaturated fats (UFA) in the diet induces a specific lipidomic signature characterized by reductions in specific ceramides and other lipid species, a pattern associated with a substantially lower risk of cardiovascular disease (-32%) and type 2 diabetes (-26%) [33]. This highlights the role of specific phospholipids and their metabolites as mediators between diet and disease.
Furthermore, individual phospholipid classes are implicated in specific pathologies:
- Sphingomyelin and Atherosclerosis: The metabolism of SM generates ceramide, a pro-apoptotic and pro-inflammatory signaling molecule. High SM intake or aberrant SM metabolism may contribute to insulin resistance and atherosclerosis through ceramide-mediated pathways [31].
- Phosphatidylserine and Cognitive Function: While some studies suggest PS supplementation may support cognitive function, authoritative bodies like the European Food Safety Authority (EFSA) have found the evidence inconclusive, noting that source (soy vs. bovine brain) may significantly influence biological activity [32].
- Phosphatidylethanolamine and Prion Diseases: PE has been shown to play a unique role in facilitating the propagation of infectious prions in the absence of nucleic acids, highlighting its significance in neurodegenerative diseases [30].
Nutritional Significance and Dietary Recommendations
Phospholipids, though comprising a smaller fraction of dietary lipids compared to triglycerides, are essential for health. They are integral components of cell membranes and contribute to cellular signaling. While the body can synthesize most phospholipids, dietary intake contributes to the body's pools.
Key Nutritional Considerations:
- Essential Fatty Acid Carrier: Phospholipids are important carriers of essential fatty acids like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Studies show that increasing dietary phospholipids elevates the EPA and DHA content in tissues [34].
- Flavor and Texture: As natural emulsifiers, phospholipids like lecithin (rich in PC) are critical in food processing for creating smooth textures, preventing separation, and enhancing mouthfeel. They also influence the generation of desirable flavor compounds during food processing and cooking [34] [27].
- Safety and Supplements: Phospholipid supplements, particularly PS and PC, are marketed for cognitive and liver health. Initial PS supplements derived from bovine brain cortex were replaced by soy-based sources due to concerns about transmissible spongiform encephalopathies [32]. Generally, phospholipid supplementation is considered safe at recommended doses.
Table 3: Phospholipid Content in Select Foods (mg/100 g) [32]
| Food Source | Phosphatidylserine (PS) | Food Source | Phospholipid Association |
|---|---|---|---|
| Soy Lecithin | ~1,650 mg | Egg Yolk | Rich in PC and PE [29] [30] |
| Atlantic Mackerel | ~480 mg | Bovine Brain | Historically a key source of PS and SM [31] [32] |
| Chicken Heart | ~414 mg | Dairy Milk | Contains SM and PC [31] |
| Chicken Liver | ~123 mg | Meat and Poultry | Contains all major classes (PC, PE, PS, SM) |
| White Beans | ~107 mg | Fish Roe | Exceptionally high in PC and other phospholipids |
| Beef | ~69 mg |
Within the realm of lipid biochemistry, triglycerides and phospholipids represent two fundamental classes of molecules with distinct biological roles and structural manifestations. Triglycerides (TAGs) serve as the primary storage form of metabolic energy in intracellular lipid droplets, characterized by a unique core-shell architecture where a neutral lipid core is surrounded by a phospholipid monolayer [35]. In contrast, phospholipids spontaneously form the phospholipid bilayer that constitutes the primary structural matrix of all cellular membranes, creating stable barriers between aqueous compartments [36] [37]. This whitepaper provides an in-depth technical examination of the spatial arrangements and self-assembly phenomena governing these structures, with particular focus on triglyceride crystalline polymorphs and their relationship to the phospholipid bilayer. Framed within research on dietary triglycerides and phospholipids, this analysis aims to equip researchers and drug development professionals with advanced methodological frameworks for investigating these crucial nanoscale assemblies.
The molecular structure of triglycerides consists of a glycerol backbone esterified to three fatty acid chains, which can vary in length, degree of saturation, and positional distribution on the glycerol molecule [8]. These structural variations directly influence the packing efficiency and polymorphic behavior of triglyceride crystals. Phospholipids, featuring a glycerol backbone with two fatty acid chains and a hydrophilic phosphate-containing headgroup, are amphipathic molecules that spontaneously self-assemble into bilayers in aqueous environments through the hydrophobic effect [36]. This fundamental difference in molecular architecture dictates their divergent biological functions: triglycerides as energy-dense storage depots and phospholipids as structural membrane components.
Structural Principles and Molecular Organization
The Phospholipid Bilayer: A Foundation for Cellular Compartmentalization
The phospholipid bilayer forms the fundamental permeability barrier of cell membranes, with a typical thickness of 5-6 nm [36]. This nanostructure emerges from the amphipathic nature of phospholipid molecules, which drives their self-assembly into two-dimensional sheets with hydrophobic cores facing inward and hydrophilic headgroups interfacing with aqueous environments. According to the fluid mosaic model proposed by Singer and Nicolson, biological membranes function as two-dimensional fluids wherein proteins are embedded within the lipid bilayer matrix [37]. Several critical features define bilayer organization:
Molecular asymmetry: The inner and outer leaflets differ in phospholipid composition, with phosphatidylcholine and sphingomyelin predominantly in the outer leaflet, and phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol concentrated in the inner leaflet [36] [37]. This asymmetry is maintained by ATP-dependent flippases and scramblases and has functional consequences for membrane signaling and apoptosis.
Phase behavior: Bilayers undergo thermotropic phase transitions from gel to fluid states, influenced by fatty acid chain length, unsaturation, and cholesterol content [36]. The introduction of double bonds creates kinks in hydrocarbon chains that disrupt packing and increase fluidity.
Lateral heterogeneity: Membrane domains known as "lipid rafts" enriched in cholesterol and sphingolipids form platforms for protein assembly and signaling [37].
Table 1: Major Phospholipid Classes in Mammalian Cell Membranes and Their Properties
| Phospholipid Class | Primary Location | Charge at pH 7 | Approximate Percentage of Total Lipid | Functional Significance |
|---|---|---|---|---|
| Phosphatidylcholine (PC) | Outer leaflet | Zwitterionic | ~50% | Structural backbone of membrane |
| Phosphatidylethanolamine (PE) | Inner leaflet | Zwitterionic | ~20-25% | Membrane fusion, curvature |
| Phosphatidylserine (PS) | Inner leaflet | Negative | ~5-10% | Apoptosis signal, signaling scaffold |
| Phosphatidylinositol (PI) | Inner leaflet | Negative | <5% | Intracellular signaling |
| Sphingomyelin | Outer leaflet | Zwitterionic | ~10-15% | Lipid rafts, signaling |
Triglyceride Crystalline Polymorphs: Structural Complexity in Energy Storage
Triglycerides exhibit complex polymorphic behavior, crystallizing in multiple distinct forms with different thermodynamic stabilities and physical properties. This polymorphism arises from variations in molecular packing and chain alignment, which are influenced by processing conditions and thermal history [8]. The primary polymorphic forms include:
α form: The least stable polymorph characterized by hexagonal hydrocarbon chain packing with minimal rotational order. This form has the lowest density and melting point.
β' form: An intermediate metastable polymorph with orthorhombic perpendicular chain packing. This form is important in food applications for its desirable texture properties.
β form: The most stable and densely packed polymorph with triclinic parallel chain stacking. This form has the highest melting point and thermodynamic stability.
The specific polymorph that forms depends on the homologous structure of the triglyceride, including fatty acid chain length, saturation patterns, and positional distribution on the glycerol backbone [8]. For example, symmetrical monounsaturated triglycerides like POP (palmitic-oleic-palmitic) exhibit different polymorphic tendencies than asymmetrical or fully saturated analogues.
Table 2: Characteristics of Primary Triglyceride Polymorphic Forms
| Polymorph | Chain Packing Structure | Subcell Type | Relative Stability | Melting Temperature | Density |
|---|---|---|---|---|---|
| α | Hexagonal | H | Lowest | Lowest | Lowest |
| β' | Orthorhombic perpendicular | O⟂ | Intermediate | Intermediate | Intermediate |
| β | Triclinic parallel | T∕∕ | Highest | Highest | Highest |
Experimental Methodologies for Structural Analysis
X-ray Scattering Techniques for Nanostructural Characterization
X-ray scattering methods provide powerful tools for investigating the nanostructural features of both triglyceride crystals and phospholipid assemblies. These techniques enable researchers to determine key structural parameters non-invasively under various environmental conditions [8]:
Small-Angle X-ray Scattering (SAXS) probes long-range ordering in the range of 1-100 nm, providing information about:
- Lamellar repeat distances in phospholipid bilayers and triglyceride crystals
- Bilayer thickness and electron density profiles
- Phase behavior and structural transitions
Wide-Angle X-ray Scattering (WAXS) investigates short-range molecular ordering in the range of 0.1-1 nm, revealing:
- Chain packing arrangements and subcell structures
- Polymorphic identity in triglyceride crystals
- Phase transitions and crystallinity
For triglyceride analysis, the combination of SAXS and WAXS enables comprehensive characterization of polymorphic behavior. SAXS measures the long spacing corresponding to the lamellar repeat distance of triglyceride molecules stacked in bilayers, while WAXS determines the short spacing reflecting the lateral packing of hydrocarbon chains [8]. Recent advances in ultra-small angle X-ray scattering (USAXS) extend these measurements to larger length scales, enabling investigation of TAG crystallite aggregation and microstructure development.
Molecular Dynamics Simulations of Lipid Assemblies
Molecular dynamics (MD) simulations provide atomic-level insights into the structure, dynamics, and thermodynamics of lipid assemblies. Both atomistic and coarse-grained approaches have been employed to investigate unique properties of lipid droplet surfaces and bilayer membranes [35]. Key methodological considerations include:
System Setup for Lipid Droplet Simulations:
- Construction of trilayer systems with core triglycerides (e.g., triolein), phospholipid monolayers (e.g., POPC), and water molecules
- Parameterization of triglyceride molecules using united-atom or all-atom force fields
- Application of surface tension to model physiological conditions
Analysis Protocols:
- Calculation of lipid-packing defects by quantifying voids at the lipid-water interface
- Determination of interdigitation between surface phospholipids and core neutral lipids using overlap parameters
- Evaluation of lateral pressure profiles across the interface
- Classification of triglyceride conformations based on orientation vectors
MD simulations have revealed that the unique surface properties of lipid droplets originate from interdigitation between surface phospholipids and core triglycerides, which affects both lipid-packing defects and the lateral pressure profile [35]. This property is extremely sensitive to membrane undulations and shows a bimodal behavior at surface tensions greater than 10 mN/m.
Comparative Analysis of Structural Properties
Molecular Packing and Interdigitation Phenomena
A fundamental distinction between triglyceride-rich structures and phospholipid bilayers lies in their molecular packing arrangements. In phospholipid bilayers, the two leaflets are largely independent, with limited interdigitation between opposing fatty acid chains [36]. In contrast, lipid droplets exhibit significant interdigitation between surface phospholipids and core triglycerides, which modulates their surface properties and protein binding characteristics [35].
Quantitative analysis of interdigitation can be performed using an overlap parameter (ρov) calculated from density profiles of triglycerides and phospholipids:
The degree of interdigitation (λov) is then determined by integrating this quantity across the interface [35]. This interdigitation profoundly influences surface properties by:
- Modulating lipid-packing defects that accommodate amphipathic helices of targeting proteins
- Affecting the lateral pressure profile at the lipid droplet surface
- Creating a bimodal response to surface tension changes
Therapeutic Targeting of Triglyceride Metabolism
Recent advances in understanding triglyceride structure and metabolism have enabled novel therapeutic approaches for managing hypertriglyceridemia and associated cardiovascular risks. Two promising strategies have emerged in clinical development:
Olezarsen is an antisense oligonucleotide that targets apolipoprotein C-III (apoC-III) mRNA, reducing its production and enhancing triglyceride clearance. In the ESSENCE-TIMI 73b phase III trial, olezarsen administered as monthly subcutaneous injections (50 mg or 80 mg) significantly reduced triglyceride levels by approximately 60% at 6 months compared to placebo [16]. More than 80% of patients achieved normal triglyceride levels (<150 mg/dL) with olezarsen treatment at 12 months, demonstrating its potent triglyceride-lowering effects.
DR10624 represents a first-in-class medication that activates FGF21, glucagon, and GLP-1 receptors, providing a multi-factorial approach to triglyceride reduction. In a 12-week phase II trial involving patients with severe hypertriglyceridemia (500-2,000 mg/dL), DR10624 administered as weekly subcutaneous injections (12.5 mg, 25 mg, or 50 mg titration) reduced triglyceride levels by 66-75% and liver fat by 63.5% [38]. Nearly 90% of patients receiving DR10624 achieved triglyceride levels below 500 mg/dL, compared to 25% in the placebo group.
Table 3: Emerging Therapies for Severe Hypertriglyceridemia
| Therapeutic Agent | Mechanism of Action | Administration | Triglyceride Reduction | Additional Effects |
|---|---|---|---|---|
| Olezarsen | Apolipoprotein C-III mRNA antagonist | Monthly subcutaneous injection (50 mg, 80 mg) | ~60% at 6 months | Reduced remnant cholesterol, non-HDL-C, apolipoprotein B |
| DR10624 | FGF21, glucagon, GLP-1 receptor agonist | Weekly subcutaneous injection (12.5 mg, 25 mg, 50 mg) | 66-75% at 12 weeks | 63.5% reduction in liver fat |
The Scientist's Toolkit: Essential Research Reagents and Methodologies
Key Research Reagent Solutions
Table 4: Essential Reagents for Investigating Lipid Assemblies
| Reagent/Material | Function/Application | Technical Specifications | Experimental Considerations |
|---|---|---|---|
| Palmitoyloleoylphosphatidylcholine (POPC) | Model phospholipid for bilayer and monolayer studies | Synthetic phospholipid with one saturated (C16:0) and one unsaturated (C18:1) chain | Maintain nitrogen atmosphere to prevent oxidation of unsaturated chains |
| Triolein (TO) | Model triglyceride for lipid droplet simulations | Glycerol triester with three oleic acid chains (C18:1) | High purity essential for consistent crystallization behavior |
| Berger Force Field | United-atom molecular dynamics parameters for lipids | Optimized for phospholipids and triglycerides | Includes corrections for double bond geometry in unsaturated chains |
| Electropermanent Magnets | Directed self-assembly of macro-scale structures | Controllable bonding actuation for spatial organization | Used in TESSERAE platform for autonomous self-assembly [39] |
| Polyacrylic Acid (PAA)-grafated Nanoparticles | Surface-mediated molecular self-assembly | Creates proton-rich microdomains for directed hydrogelation | Brush length (156-175 nm) and concentration affect assembly kinetics [40] |
Experimental Workflow for Lipid Droplet Characterization
The following diagram illustrates a comprehensive experimental workflow for characterizing triglyceride crystalline structures and their relationship to phospholipid monolayer organization in lipid droplets:
Molecular Organization in Lipid Assemblies
The structural relationships between triglyceride polymorphs and phospholipid bilayers can be visualized through their molecular organization principles:
The spatial arrangements and self-assembly behaviors of triglycerides and phospholipids represent sophisticated paradigms of molecular organization with profound implications for both basic biology and therapeutic development. The structural principles governing triglyceride crystalline polymorphs—with their complex lamellar stacking and polymorphic transitions—contrast with the fluid mosaic organization of phospholipid bilayers, yet both systems share fundamental physical-chemical drivers rooted in hydrophobic effects and molecular packing constraints.
Recent advances in therapeutic targeting of triglyceride metabolism, particularly through apolipoprotein C-III inhibition with olezarsen and multi-receptor agonism with DR10624, demonstrate the translational potential of understanding these structural principles at a molecular level [38] [16]. Similarly, emerging techniques in directed self-assembly using polymer brush-functionalized nanoparticles [40] and computational approaches using molecular dynamics simulations [35] provide powerful methodologies for investigating and manipulating these nanostructures.
Future research directions will likely focus on integrating multi-scale approaches that connect molecular-level packing phenomena to macroscopic material properties and biological functions. The development of more sophisticated force fields for molecular simulations, advances in time-resolved X-ray scattering methodologies, and innovative surface-assisted assembly techniques will further enhance our understanding of these fundamental biological structures and enable novel therapeutic strategies for managing lipid-associated disorders.
Lipids are essential components of human nutrition, serving as critical structural elements of cell membranes, concentrated energy sources, and precursors for bioactive molecules. Within this broad class, triglycerides (TGs) and phospholipids (PLs) represent two fundamentally important categories with distinct chemical structures, dietary origins, and metabolic fates. The chemical structure of these lipids—specifically the backbone, fatty acid composition, and polar head groups—profoundly influences their digestion, absorption, transport, and ultimate biological activity. This whitepaper provides a comprehensive technical examination of the dietary sources and bioavailability of triglycerides and phospholipids, framed within ongoing research into their chemical structure-bioactivity relationships. For researchers and drug development professionals, understanding these nuances is critical for designing effective lipid-based therapeutics and nutritional interventions.
Chemical Structure and Fundamentals
The fundamental structural differences between triglycerides and phospholipids dictate their divergent biological roles and metabolic pathways.
Triglyceride Structure: TGs consist of a three-carbon glycerol backbone esterified to three fatty acid molecules [19] [28]. These fatty acids can be saturated, monounsaturated, or polyunsaturated, with varying chain lengths that influence the triglyceride's physical properties (e.g., melting point) and metabolic characteristics [19]. TGs are highly nonpolar and serve as the body's primary form of energy storage, comprising more than 95% of dietary lipids [19] [23].
Phospholipid Structure: PLs share a glycerol backbone with TGs but are typically diacyl molecules, with two fatty acid chains and a phosphate-containing polar head group attached to the third carbon [41]. This amphipathic nature—possessing both hydrophobic and hydrophilic regions—makes PLs ideal as primary structural components of cellular membranes [28] [41]. Common phospholipids include phosphatidylcholine (PC), phosphatidylethanolamine, and phosphatidylserine, which differ in their headgroup compositions [42].
The following diagram illustrates the core structural differences and the enhanced bioavailability pathway of phospholipid-bound fatty acids, particularly docosahexaenoic acid (DHA).
Figure 1: Structural and Bioavailability Pathways of Triglycerides vs. Phospholipids. The presence of a polar head group in phospholipids facilitates distinct digestive processing and cellular uptake mechanisms, culminating in the enhanced bioavailability of phospholipid-bound fatty acids like DHA [26] [41].
Triglycerides are the predominant form of dietary fat. Most foods contain a mixture of fatty acid types, but can be categorized by their predominant fatty acid profile [19].
Table 1: Dietary Sources of Triglycerides and Predominant Fatty Acid Composition
| Source Category | Example Foods | Predominant Fatty Acid Type(s) | Notable Features |
|---|---|---|---|
| Saturated Fat-Rich | Red meat, butter, cheese, cocoa butter, coconut oil, palm oil [19] | Saturated Fatty Acids (SFAs) | Solid at room temperature; tightly packed straight chains [19]. |
| Monounsaturated Fat-Rich | Olive oil, avocados, peanuts, almonds, chicken, turkey [19] | Monounsaturated Fatty Acids (MUFAs) | Liquid (oils) at room temperature; one cis double bond creates a kink [19]. |
| Polyunsaturated Fat-Rich | Soybean oil, corn oil, safflower oil, walnuts, whole grains, seafood [19] | Polyunsaturated Fatty Acids (PUFAs) | Liquid at room temperature; multiple double bonds. Include essential fatty acids (linoleic and α-linolenic acid) [19]. |
| Omega-3 PUFA Rich | Flaxseeds, chia seeds, walnuts, canola oil [19] | Alpha-linolenic Acid (ALA, 18:3 n-3) | ALA is a precursor to EPA and DHA, but human conversion is inefficient (<10%) [19] [26]. |
| Marine Omega-3 Rich | Fatty fish (salmon, mackerel, sardines, tuna) [19] [43] | Eicosapentaenoic Acid (EPA, 20:5 n-3) and Docosahexaenoic Acid (DHA, 22:6 n-3) | Provides pre-formed EPA and DHA, primarily in the triglyceride form (TAG-DHA) [26]. |
Phospholipids are consumed in smaller quantities than triglycerides and are often found in complex food matrices.
Table 2: Dietary and Commercial Sources of Phospholipids
| Source Category | Example Sources | Predominant Phospholipid Forms & Fatty Acids | Notable Features |
|---|---|---|---|
| Animal-Based | Egg yolks, liver, meat, fish roe [26] [23] | Phosphatidylcholine (PC), Phosphatidylethanolamine (PE) | Major dietary source of phosphatidylcholine in typical diets [23]. |
| Marine PL-Rich | Antarctic krill (Euphausia superba), fish roe [26] [44] | PC with EPA and DHA (PL-DHA/PL-EPA) | Krill oil typically contains 40-45% of its omega-3s as PLs, often with an EPA:DHA ratio of 1.2:1 to 1.8:1 [26] [44]. |
| Plant-Based | Soybeans, sunflower seeds, canola | PC, Phosphatidylinositol (PI) | Soy lecithin is a common commercial source used in food and supplements. |
| Enzymatically Engineered | Synthesized lysophospholipids, structured phospholipids [26] | Tailored PL species (e.g., sn-2 DHA) | Emerging technology for producing PLs with specific fatty acids at the sn-2 position to optimize bioavailability [26]. |
Bioavailability and Metabolic Pathways
The molecular form of a lipid—whether it is esterified as a triglyceride or a phospholipid—significantly impacts its metabolic disposition and biological efficacy.
Bioavailability of Triglycerides
The absorption of dietary triglycerides is a highly efficient process, with the body utilizing more than 95% of consumed TGs [23]. The pathway involves:
- Digestion: In the small intestine, pancreatic lipase hydrolyzes TGs at the sn-1 and sn-3 positions, producing free fatty acids (FFAs) and 2-monoacylglycerol (2-MAG) [28].
- Micellization: These lipolytic products are incorporated into mixed micelles with the aid of bile salts, which ferry them to the intestinal brush border for absorption [28] [43].
- Re-synthesis and Packaging: Inside the enterocytes, FFAs and 2-MAG are re-esterified into new triglycerides. These TGs are then packaged, along with cholesterol and apolipoprotein B-48, into chylomicrons [28].
- Systemic Transport: Chylomicrons are secreted into the lymphatic system before entering the bloodstream, where lipoprotein lipase (LPL) on capillary endothelial cells hydrolyzes their core TGs to release FFAs for tissue uptake or storage [28].
Enhanced Bioavailability of Phospholipids
A growing body of evidence indicates that long-chain polyunsaturated fatty acids (LC-PUFAs) like DHA and EPA are more bioavailable when delivered as phospholipids compared to triglycerides [26] [45] [44].
- Mechanisms for Enhanced Bioavailability:
- Efficient Cellular Uptake: Phospholipids, particularly phosphatidylcholine, are intrinsic components of cell membranes. This similarity may facilitate the passage of their constituent fatty acids through the intestinal wall [44].
- Distinct Transport Pathways: PL-DHA can be absorbed as lysophospholipids and is a preferred substrate for the Mfsd2a transporter, which is critical for the efficient uptake of DHA across the blood-brain barrier [26].
- Bypassing Hepatic Metabolism: Some phospholipid forms may be directly absorbed into the lymphatic system via a process that bypasses initial hepatic metabolism, potentially leading to more efficient delivery to peripheral tissues [26].
- Resistance to Pancreatic Lipase: The phospholipid structure is less susceptible to hydrolysis by pancreatic lipase, which may protect the LC-PUFAs from oxidation and preserve their structural integrity during digestion [43].
Experimental Evidence from Comparative Studies
Table 3: Key Findings from Bioavailability and Efficacy Studies
| Study Model | Intervention Comparison | Key Findings | Reference |
|---|---|---|---|
| Human RCT (Ulven et al.) | Krill Oil (PL) vs. Fish Oil (TG) providing equal EPA+DHA | Higher incorporation of EPA and DHA into plasma phospholipids after krill oil intake. | [44] |
| Mouse Model of Obesity | DHA/EPA as PL vs. TG in high-fat diet | PL form was superior in preventing glucose intolerance, reducing hepatosteatosis and WAT inflammation. Correlated with better tissue DHA accretion. | [45] |
| Postprandial Human Study | Krill Oil (PL) vs. Fish Oil (TG) | Higher plasma EPA/DHA levels and different pharmacokinetic profile after krill oil consumption, suggesting enhanced bioavailability. | [44] |
| In Vitro & Animal Models | PL-DHA with sn-2 DHA | Position-specific effects: sn-2 DHA maintains a hairpin conformation for optimal enzymatic recognition and membrane incorporation. | [26] |
Experimental Protocols for Bioavailability Assessment
To evaluate the bioavailability and metabolic effects of different lipid forms, researchers employ a suite of standardized protocols.
Postprandial Lipid Response Trial
This clinical trial design is a cornerstone for assessing the acute bioavailability of lipids and fat-soluble micronutrients [46].
- Protocol Overview: A randomized, open-label, crossover postprandial trial is conducted.
- Subject Selection: Enroll healthy subjects (e.g., n=12) following ethical approval and informed consent.
- Intervention: Participants consume test foods or supplements (e.g., krill oil PL vs. fish oil TG) in random order after a 12-hour fast, with a sufficient washout period between interventions [46] [44].
- Blood Sampling: Serial blood samples are collected at baseline and at regular intervals post-consumption (e.g., 1, 2, 3, 4, 5, 6 hours).
- Bioavailability Analysis:
- Triglyceride Response: Measure chylomicron-TG concentration in plasma or isolated chylomicron fractions [46].
- Fatty Acid Incorporation: Analyze the area under the curve (AUC) for the increase in target fatty acids (e.g., EPA, DHA) in plasma phospholipids or red blood cell (RBC) membranes [44].
- Time to Peak (T~max~): Record the time taken to reach the maximum concentration (C~max~) for different lipid forms [46].
Tissue-Specific Isotope Tracer Studies
For detailed metabolic tracing, advanced techniques are used.
- Protocol Overview: This method tracks the fate of isotopically labeled fatty acids administered in different molecular forms (TG vs. PL).
- Tracer Administration: Subjects or animal models receive stable isotope-labeled (e.g., ^13^C) DHA or EPA as a triglyceride or phospholipid.
- Tissue Sampling and Analysis: Collect target tissues (e.g., brain, liver, adipose) after a predetermined period.
- Metabolic Fate Assessment: Use gas chromatography-mass spectrometry (GC-MS) to quantify the incorporation of the labeled fatty acids into specific tissue lipid pools (e.g., membrane PLs, TGs) [26]. This allows for the determination of tissue-specific uptake and metabolic partitioning.
The Scientist's Toolkit: Key Research Reagents and Materials
Table 4: Essential Reagents and Materials for Lipid Bioavailability Research
| Reagent / Material | Function and Application in Research |
|---|---|
| Krill Oil Extract | A natural source of omega-3 fatty acids (EPA and DHA) esterified predominantly to phospholipids. Used as an experimental intervention to study the effects of PL-DHA/EPA [26] [44]. |
| Fish Oil Concentrates | A source of omega-3 fatty acids (EPA and DHA) primarily in the triglyceride form. Serves as the standard comparator for bioavailability and efficacy studies [45] [44]. |
| Stable Isotope-Labeled Fatty Acids (e.g., ^13^C-DHA, ^2^H-EPA) | Tracers to quantitatively monitor the absorption, tissue distribution, and metabolic fate of fatty acids from different molecular forms (TG vs. PL) in vivo [26]. |
| Phospholipase A₂ (PLA₂) | Enzyme used to hydrolyze the sn-2 fatty acid from phospholipids for analysis or to create lysophospholipids. Also a key enzyme in phospholipid digestion [26]. |
| Lipoprotein Lipase (LPL) | Critical enzyme in triglyceride metabolism; hydrolyzes TG core of chylomicrons and VLDL to release free fatty acids for tissue uptake. Used in enzymatic assays [28]. |
| Mfsd2a Transporter Assays | Cell-based or in vitro systems expressing the Mfsd2a transporter to specifically study the uptake mechanism of lysophospholipid-DHA into the brain and other tissues [26]. |
| Chylomicron Isolation Kits | Reagents for ultracentrifugation or immunoaffinity methods to isolate chylomicrons from postprandial plasma for direct measurement of TG and lipid-soluble micronutrient transport [46]. |
The chemical structure of dietary lipids is a primary determinant of their bioavailability and physiological impact. While triglycerides serve as efficient energy vectors, phospholipids offer a superior delivery system for bioactive fatty acids, particularly for targeting neural tissues and modulating metabolic and inflammatory pathways. The evidence demonstrates that PL-bound DHA exhibits enhanced bioavailability and distinct tissue distribution compared to its TG-bound counterpart, mediated by specific transport mechanisms like Mfsd2a. For researchers and pharmaceutical developers, these insights are transformative. They underscore the potential of designing structured phospholipids and employing advanced delivery systems to optimize the therapeutic efficacy of lipid-based compounds. Future research focusing on the position-specific effects of fatty acids within the phospholipid molecule and their long-term health outcomes will further refine our ability to harness these molecules for improved human health.
Analytical and Applied Frontiers: Characterizing Lipids and Engineering Functional Systems
Lipids, fundamental to human nutrition and health, serve as critical structural components of cell membranes and as energy stores. The chemical structure of dietary triglycerides and phospholipids directly dictates their biological function and metabolic fate. This whitepaper details two advanced analytical techniques essential for modern lipid research: High-Performance Liquid Chromatography with Evaporative Light Scattering Detection (HPLC-ELSD) for phospholipid profiling and X-ray Scattering for investigating triglyceride polymorphs. These methodologies provide researchers, scientists, and drug development professionals with powerful tools to deconstruct the intricate architecture of lipids, enabling advances in nutritional science, pharmaceutical formulation, and functional food development. The integration of these techniques into a coherent analytical framework allows for a comprehensive understanding of lipid behavior from the molecular to the mesoscale level, providing critical insights for a broad thesis on dietary lipid research.
HPLC-ELSD for Phospholipid Profiling
Principles and Applications
Phospholipids are vital amphiphilic molecules, forming the backbone of biological membranes and acting as key signaling molecules. Their analysis is crucial for understanding cell membrane integrity, lipoprotein metabolism, and the development of lipid-based drug delivery systems. HPLC-ELSD combines the superior separation capabilities of high-performance liquid chromatography with the universal detection properties of an evaporative light scattering detector. This technique is particularly valuable for phospholipids, which often lack strong chromophores, making UV detection less sensitive. The ELSD operates by nebulizing the chromatographic eluent, evaporating the mobile phase, and detecting the non-volatile analyte particles via light scattering, offering a robust and sensitive solution for quantifying complex phospholipid mixtures [47] [48].
A significant application of HPLC-ELSD is the specific detection and quantification of ether phospholipids in human plasma, which are implicated in conditions like Alzheimer's disease and metabolic syndrome. By pre-treating plasma with phospholipase A1 (PLA1), which hydrolyzes diacyl phospholipids but leaves ether bonds intact, researchers can isolate and accurately measure ethanolamine (eEtnGpl) and choline (eChoGpl) ether phospholipids as independent peaks via HPLC-ELSD. This method provides a accessible alternative to more expensive LC/MS/MS systems for clinical laboratories [48].
Experimental Protocol: HPLC-ELSD for Phospholipid Classes
1. Sample Preparation (Plasma Ether Phospholipids):
- Obtain human plasma via venipuncture, using heparin as an anticoagulant. Separate plasma by centrifugation at 1,000× g for 5 minutes [48].
- Treat 80 µL of plasma with 20 µL of PLA1 (from Thermomyces lanuginosus, diluted 1:1 with 0.1 M citrate buffer, pH 4.5) [48].
- Incubate the mixture at 45°C for 60 minutes to ensure complete hydrolysis of diacyl phospholipids [48].
- Perform lipid extraction by adding 800 µL of n-hexane/isopropanol (3:2, v/v) to the PLA1-treated plasma. Vortex vigorously and place in an ultrasound bath for 5 minutes [48].
- Add 400 µL of sodium sulfate solution (1 g anhydrous Na2SO4 in 15 mL water), mix, and allow phases to separate for 5 minutes [48].
- Recover 400 µL of the upper hexane layer. Re-extract the lower phase with 400 µL of hexane/isopropanol (7:2, v/v) and combine the hexane layers [48].
- Dry the combined organic extracts under a stream of nitrogen gas. Reconstitute the dried lipid extract in 200 µL of hexane/isopropanol (3:2, v/v) prior to HPLC injection [48].
2. HPLC-ELSD Analysis:
- Column: Use a normal-phase column, such as a LiChrosphere 100 Diol column (250 × 2 mm, 5 µm particle size) [48].
- Mobile Phase: Utilize a binary gradient system [48]:
- Mobile Phase A: n-Hexane/2-propanol/acetic acid (82:17:1, v/v/v) with 0.08% triethylamine (TEA).
- Mobile Phase B: 2-Propanol/water/acetic acid (85:14:1, v/v/v) with 0.08% TEA.
- Gradient Program: [48]
- Begin at 4% Mobile Phase B.
- Increase linearly to 37% B over 21 minutes.
- Ramp to 85% B over 4 minutes and hold for 1 minute.
- Return to 4% B over 3 minutes and re-equilibrate for 7 minutes.
- Flow Rate: 0.4 mL/min [48].
- Column Temperature: Maintain at 50°C [48].
- ELSD Parameters: Set evaporation temperature to 60°C, nebulizer temperature to 30°C, nitrogen gas flow rate to 1.0 L/min, and sensitivity gain to 6 [48].
- Injection Volume: 20 µL of the reconstituted lipid extract [48].
3. Data Analysis:
- Identify phospholipid classes (e.g., phosphatidylcholine, phosphatidylethanolamine, sphingomyelin, and ether phospholipids) based on retention times compared to authentic standards [47] [48].
- Construct calibration curves (typically 5-40 µg) for each phospholipid class of interest. The ELSD response is generally linear within this range, with detection limits below 1 µg for major classes [47].
Figure 1: HPLC-ELSD phospholipid analysis workflow.
Research Reagent Solutions
Table 1: Essential reagents for HPLC-ELSD phospholipid analysis.
| Reagent/Consumable | Function/Application | Exemplary Specification |
|---|---|---|
| Phospholipase A1 (PLA1) | Selective hydrolysis of diacyl phospholipids for ether phospholipid analysis | From Thermomyces lanuginosus, diluted in 0.1 M citrate buffer (pH 4.5) [48] |
| Normal-Phase HPLC Column | Separation of phospholipid classes by polarity | LiChrosphere 100 Diol (250 × 2 mm, 5 µm) [48] |
| HPLC Solvents | Mobile phase components for gradient elution | n-Hexane, 2-propanol, acetic acid, triethylamine (HPLC grade) [48] |
| Phospholipid Standards | Qualitative identification and quantitative calibration | Phosphatidylcholine, phosphatidylethanolamine from natural sources [48] |
X-ray Scattering for Triglyceride Polymorphs
Principles and Polymorph Characterization
Triglycerides (TAGs) exhibit a complex crystallization behavior known as monotropic polymorphism, forming three primary polymorphic forms: α, β', and β. These polymorphs possess distinct physicochemical properties—melting temperature, subcell lattice structure, and mass density—that profoundly influence the texture, stability, and functionality of fat-based products in food, cosmetics, and pharmaceuticals [49] [8]. X-ray scattering techniques provide a powerful, multi-scale approach to investigate these hierarchical structures, covering molecular packing, lamellar stacking, and mesoscale aggregation [8] [50].
The polymorphic forms are identified by their unique X-ray scattering signatures:
- α Form: A single strong reflection in the wide-angle region (WAXS) at approximately 4.15 Å (q ≈ 15.1 nm⁻¹) and a long spacing (SAXS) that typically corresponds to a two-chainlength (2L) structure [49] [8].
- β' Form: Characterized by two strong short spacings in WAXS at around 3.8 and 4.2 Å, often exhibiting a 2L lamellar structure [49].
- β Form: The most stable polymorph, shows a strong short spacing at approximately 4.6 Å and often transitions to a triple-chainlength (3L) structure in monoacid saturated TAGs [49] [8].
Table 2: Characteristic X-ray scattering parameters for triglyceride polymorphs [49] [8].
| Polymorph | Short Spacings (Å) [WAXS] | Long Spacing (Å) [SAXS] | Chain Packing Subcell | Thermodynamic Stability |
|---|---|---|---|---|
| α | ~4.15 (single strong reflection) | ~40-50 (depending on TAG) | Hexagonal | Least stable |
| β' | ~3.8, 4.2 (doublet) | ~40-50 (depending on TAG) | Orthorhombic perpendicular | Intermediate |
| β | ~4.6 (strong), ~3.8, 3.9 (weaker) | ~60-75 (3L structure for monoacid) | Triclinic parallel | Most stable |
Experimental Protocol: Multi-scale X-ray Scattering of Triglycerides
1. Sample Preparation:
- For bulk fat systems, prepare 30% dilutions of solid fats (e.g., palm stearin or fully hydrogenated rapeseed oil) in liquid oils (e.g., high oleic sunflower oil or triolein) to model real fat systems [51] [50].
- Melt samples completely to erase crystal memory, then control cooling and crystallization using a temperature-controlled stage (e.g., Peltier device) [51].
- For time-resolved studies, maintain precise temperature control throughout the experiment to capture polymorphic transitions [51].
2. Combined DSC and X-ray Scattering:
- Utilize a setup that combines Differential Scanning Calorimetry (DSC) with simultaneous X-ray scattering to correlate thermal events with structural changes [51].
- Perform experiments at synchrotron facilities for high-intensity X-ray beams, enabling real-time, time-resolved studies of crystallization kinetics [51] [50].
3. Multi-scale X-ray Scattering:
- Wide-Angle X-ray Scattering (WAXS): Configure detector for q-range of 7-20 nm⁻¹ (d-spacing ~0.9-2.8 Å) to resolve chain packing and polymorph identity [8].
- Small-Angle X-ray Scattering (SAXS): Set up for q-range of 0.05-7 nm⁻¹ (d-spacing ~0.9-125 nm) to determine lamellar stacking and long spacings [8] [50].
- Ultra-Small-Angle X-ray Scattering (USAXS): Employ for q-range of 0.003-0.3 nm⁻¹ (length scales ~25 nm to 3.5 µm) to characterize crystalline nanoplatelets (CNPs) and their aggregation behavior [51] [50].
4. Data Analysis:
- Polymorph Identification: Identify polymorphic forms by matching WAXS peaks to known short spacings and SAXS patterns to characteristic long spacings [8].
- Crystallite Size: Apply Scherrer equation to WAXS peak broadening to estimate crystallite size [8].
- Lamellar Analysis: Calculate electron density profiles from SAXS data to decompose lamellar repeat distance into bilayer and monolayer contributions [8].
- CNP Characterization: Model USAXS data using shape-dependent models (e.g., polydisperse parallelepipeds) to determine CNP cross-section and fractal dimensionality of aggregates [50].
Figure 2: Multi-scale X-ray scattering analysis workflow for triglycerides.
Research Reagent Solutions
Table 3: Essential materials and tools for X-ray scattering of triglycerides.
| Reagent/Material | Function/Application | Exemplary Specification |
|---|---|---|
| Model Triglyceride Systems | Fundamental studies of polymorphism | Monoacid TAGs (e.g., tripalmitin, tristearin); Mixed-acid TAGs (e.g., OPO/POP) [49] [8] |
| Natural Fat Systems | Real-world application studies | Palm stearin, fully hydrogenated rapeseed oil, high oleic sunflower oil, anhydrous milk fat [51] [8] |
| Temperature Control Stage | Precise thermal management for crystallization studies | Peltier-based device with ±0.1°C accuracy [51] |
| Synchrotron Beam Access | High-intensity X-ray source for time-resolved studies | SAXS/WAXS/USAXS beamline capability [51] [50] |
Integrated Data Analysis and Interpretation
Complementary Insights for Lipid Research
The integration of HPLC-ELSD and X-ray scattering data provides a comprehensive picture of lipid structure and function across multiple length scales. While HPLC-ELSD delivers detailed molecular composition of phospholipid mixtures, X-ray scattering reveals the structural organization of triglycerides from molecular packing to mesoscale aggregation. This multi-technique approach is particularly powerful for investigating structure-function relationships in complex lipid systems such as lipoproteins, lipid nanoparticles, and processed foods.
For instance, phospholipid composition analyzed by HPLC-ELSD directly influences the crystallization behavior and polymorphic stability of triglycerides in emulsified systems. Surface-active phospholipids can inhibit or promote specific polymorphic transitions by interacting with triglyceride crystal surfaces, effects that can be quantified through time-resolved X-ray scattering [49]. Similarly, in pharmaceutical applications, the phospholipid profile of solid lipid nanoparticle (SLN) formulations determined by HPLC-ELSD correlates with structural data from X-ray scattering to optimize drug loading and release kinetics [49].
Application to Dietary Lipid Research
In the context of a broader thesis on the chemical structure of dietary triglycerides and phospholipids, these techniques enable researchers to:
- Correlate specific phospholipid classes with the absorption and metabolism of dietary fats [52] [23].
- Understand how triglyceride polymorphs influence the nutritional and sensory properties of foods [49] [8].
- Investigate the role of lipid structures in the development of lipid-based drug delivery systems [49].
- Monitor changes in lipid composition and structure during processing and storage of lipid-containing products [51] [8].
The methodological framework presented here provides a robust foundation for advancing our understanding of dietary lipids and their impact on health and disease, offering researchers a comprehensive toolkit for structural analysis of both phospholipids and triglycerides.
Medium-chain triglycerides (MCTs) represent a unique class of dietary lipids with significant therapeutic potential for metabolic and neurological disorders. Unlike their long-chain counterparts, MCTs are rapidly absorbed and efficiently metabolized by the liver, where they serve as potent ketogenic precursors even in the presence of carbohydrates. This technical review examines the molecular mechanisms through which MCTs influence metabolic health, with particular focus on their structural determinants of bioavailability, impact on hepatic gene expression via PPARα activation, and signaling pathways that underlie their cognitive benefits. Recent advances in understanding how triglyceride structure influences metabolic outcomes are discussed alongside methodological considerations for preclinical and clinical investigation. The emerging evidence positions MCTs as promising therapeutic agents for conditions ranging from Alzheimer's disease to metabolic syndrome, while highlighting important unanswered questions regarding their long-term metabolic effects.
Medium-chain triglycerides (MCTs) are composed of saturated fatty acids with 6-12 carbon atoms, primarily caprylic (C8) and capric (C10) acids. Their unique metabolic properties stem from differences in absorption and cellular handling compared to long-chain triglycerides (LCTs). While LCTs require packaging into chylomicrons and transport via lymph, MCTs are largely absorbed intact and transported directly to the liver via the portal vein [53]. In hepatocytes, medium-chain fatty acids (MCFAs) bypass the carnitine transport system required for mitochondrial import of long-chain fatty acids, leading to rapid β-oxidation and subsequent ketone body production [53].
The therapeutic potential of MCTs extends across multiple physiological domains. As ketogenic agents, they provide alternative cerebral fuel in conditions characterized by impaired glucose metabolism, such as Alzheimer's disease [54] [53]. Their effects on systemic metabolism include modulation of hepatic lipogenesis, insulin sensitivity, and energy expenditure [54]. Importantly, recent evidence suggests that MCTs may exert benefits through both ketone-dependent and ketone-independent mechanisms, including direct signaling effects of MCFAs and modulation of gene expression programs via nuclear receptors [54] [55].
Structural Determinants of MCT Bioavailability and Function
Triglyceride Structure and Digestibility
The stereospecific positioning of fatty acids on the glycerol backbone significantly influences the metabolic fate of dietary triglycerides. Research demonstrates that the triglyceride structure impacts digestibility and bioaccessibility of nutritional lipids during digestion [56]. In vitro digestion models comparing physical mixtures of medium-chain and long-chain triglycerides (PM) with structured medium- and long-chain triglycerides (MLCT) revealed significant differences in digestive parameters:
Table 1: Digestibility Parameters of Different Triglyceride Structures
| Parameter | Physical Mixture (PM) | Structured MLCT | Significance |
|---|---|---|---|
| FFA Release (%) | 92.82% | 99.88% | P < 0.05 |
| First-order Rate Constant (s⁻¹) | 0.0444 | 0.0395 | P < 0.05 |
| DHA/EPA Bioaccessibility | Lower | Higher | Not specified |
These findings demonstrate that MLCTs release more free fatty acids (99.88% vs. 92.82%, P < 0.05) despite having a lower rate constant for FFA release (0.0395 vs. 0.0444 s⁻¹, P < 0.05) compared to physical mixtures [56]. This suggests that the structural organization of fatty acids on the triglyceride molecule significantly influences both the efficiency and kinetics of digestion, with important implications for the bioaccessibility of specific fatty acids, particularly DHA and EPA which showed enhanced bioaccessibility from MLCTs [56].
The conserved structure of human milk triglycerides provides further evidence for the biological importance of fatty acid positioning. Approximately 70% of palmitic acid (16:0) in human milk is esterified at the sn-2 position, while unsaturated fatty acids like 18:1(n-9) and 18:2(n-6) are preferentially positioned at the sn-1,3 positions [57]. This configuration enables efficient absorption of palmitic acid by conserving it as sn-2 monoacylglycerols, which are absorbed, reassembled, and secreted in plasma while conserving the original milk triglyceride configuration [57].
Membrane Phospholipids and Cellular Function
Phospholipids constitute fundamental structural elements of biological membranes, with their fatty acyl chain composition significantly impacting membrane biophysical properties and biological function [58]. The three predominant phospholipids in the human brain are phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylethanolamine (PS), accounting for approximately 35-40%, 35-40%, and 20% of brain phospholipids, respectively [59]. Mitochondrial membranes contain a more diverse phospholipid profile, including cardiolipin and phosphatidylglycerol exclusively present in mitochondrial membranes [59].
The PC/PE ratio represents a critical determinant of membrane integrity and metabolic function. Abnormally high or low cellular PC/PE ratios influence energy metabolism in various organelles and are linked to disease progression [60]. Specifically, the PC/PE ratio regulates lipid, lipoprotein, and whole-body energy metabolism, with alterations observed in conditions including non-alcoholic fatty liver disease (NAFLD) [60]. The LXR-LPCAT3 signaling pathway has been identified as a key regulator of phospholipid metabolism, with LPCAT3 instrumental in maintaining systemic lipid homeostasis through roles in hepatic lipogenesis, intestinal lipid absorption, and lipoprotein secretion [58].
Molecular Mechanisms of MCT Action
Hepatic Metabolism and Ketogenesis
The therapeutic effects of MCTs are primarily mediated through their unique hepatic metabolism. Unlike long-chain fatty acids, MCFAs do not require transport proteins to cross mitochondrial membranes and undergo rapid β-oxidation independent of the carnitine shuttle system [53]. This metabolic bypass becomes particularly significant in conditions of metabolic dysregulation, as MCFA oxidation remains efficient even when malonyl-CoA inhibition restricts long-chain fatty acid entry into mitochondria [53].
When hepatic β-oxidation of MCFAs produces acetyl-CoA exceeding tricarboxylic acid (TCA) cycle capacity, the excess acetyl-CoA is redirected toward ketogenesis, producing acetoacetate (AcAc) and β-hydroxybutyrate (βHB) [53]. These ketone bodies are released into circulation and serve as alternative metabolic substrates for peripheral tissues, particularly the brain. Importantly, MCFAs are ketogenic even in a well-fed state with normal carbohydrate intake, unlike long-chain fatty acids which are primarily ketogenic under conditions of starvation, ketogenic diet, or diabetes [53].
Figure 1: MCT Metabolic Pathways - This diagram illustrates the unique metabolic fate of medium-chain triglycerides compared to long-chain triglycerides, highlighting the hepatic pathways that lead to ketone body production and alternative metabolic fates.
PPARα-Mediated Gene Regulation
MCTs and their metabolite MCFAs exert significant effects on gene expression programs, primarily through activation of the nuclear receptor PPARα (Peroxisome Proliferator-Activated Receptor Alpha). Research using synthetic triglycerides composed of single fatty acids demonstrated that unsaturated fatty acids regulate hepatic gene expression almost exclusively through PPARα-dependent mechanisms [55]. The magnitude of gene expression changes increases with fatty acid chain length and degree of unsaturation, with docosahexaenoic acid (C22:6) showing particularly potent effects [55].
PPARα activation by MCFAs leads to transcription of genes involved in fatty acid oxidation, ketogenesis, and lipid transport. This genomic mechanism complements the direct metabolic effects of MCFAs and may underlie some of the longer-term therapeutic benefits of MCT supplementation. The specificity of this pathway is demonstrated by the finding that nearly every gene regulated by dietary unsaturated fatty acids remains unaltered in PPARα knockout mice [55].
Non-Ketone Mechanisms in Cognitive Improvement
While ketone bodies have traditionally been considered the primary mediators of MCTs' cognitive benefits, emerging evidence suggests significant ketone-independent mechanisms. In mouse models of Alzheimer's disease, MCT supplementation improved hippocampal-dependent spatial learning and memory, increased dendritic spine density, and modulated expression of genes associated with mitochondrial functions, synaptic structure, and insulin signaling [54]. Remarkably, these benefits occurred without elevation of circulating ketones, suggesting direct effects of MCFAs or alternative metabolic pathways [54].
MCT supplementation enhanced peripheral insulin response in AD mice, while a ketogenic diet conversely unveiled latent metabolic vulnerability, increasing hyperglycemia, body weight gain, and adiposity [54]. Both interventions partially reversed liver metabolic abnormalities, but through distinct mechanisms: the ketogenic diet selectively triggered hepatic neutral lipid depletion and proinflammatory gene expression, while MCTs downregulated expression of cholesterol-related genes [54].
Therapeutic Applications and Efficacy
Neurological Disorders
MCT supplementation demonstrates particular promise for cognitive disorders characterized by cerebral glucose hypometabolism. Human studies show that chronic MCT supplementation improves cognitive performance in individuals with mild cognitive impairment and Alzheimer's disease [53]. The efficacy correlates with increased cerebral ketone body uptake and appears particularly beneficial for carriers of the APOE4 allele, who demonstrate reduced cerebral glucose metabolism [53].
Table 2: Clinical Studies of MCT Supplementation in Cognitive Disorders
| Study Population | MCT Dose & Composition | Duration | Key Outcomes | Correlation with Ketones |
|---|---|---|---|---|
| Mild to Moderate Dementia | 42g/day max (25.2g average), C8 | 6 months + 6 months extension | Improved cognitive scores | Not specified |
| MCI Subjects | 30g/day, C8+C10 | 6 weeks | Improved verbal fluency; Increased DAN connectivity | Correlation with brain KB uptake |
| AD Mouse Models | 10% MCT supplementation | Not specified | Improved cognition, spine density; No ketone increase | Benefits without ketone elevation |
In subjects with mild cognitive impairment, MCT supplementation (30g/day, C8+C10) for 6 weeks improved verbal fluency scores and increased functional connectivity in the dorsal attention network (DAN) by 59% compared to placebo [53]. This enhanced connectivity correlated with increased ketone body uptake, suggesting improved brain network function in regions vulnerable to Alzheimer's pathology [53].
Metabolic Health
Beyond neurological applications, MCTs demonstrate benefits for systemic metabolic health. In Alzheimer's mouse models, MCT supplementation enhanced peripheral insulin response, suggesting potential applications for insulin resistance and metabolic syndrome [54]. The distinct metabolic effects of MCTs compared to ketogenic diets are particularly noteworthy: while both interventions improved cognition and synaptic markers, MCTs did not induce the metabolic disturbances observed with ketogenic diets, including hyperglycemia and excessive weight gain [54].
The impact of MCTs on hepatic metabolism includes modulation of genes involved in cholesterol homeostasis and reduction of lipid droplet accumulation [54]. These effects suggest potential applications for non-alcoholic fatty liver disease (NAFLD), particularly given the established role of phospholipid metabolism in this condition through the LXR-LPCAT3 pathway [58].
Experimental Methodologies and Research Protocols
In Vitro Digestion Models
Standardized in vitro digestion models provide valuable tools for investigating the digestibility and bioaccessibility of different triglyceride structures. The following protocol adapted from Wang et al. [56] allows quantitative comparison of digestive parameters:
Objective: To evaluate the effects of triglyceride structure on digestibility and bioaccessibility during simulated gastrointestinal digestion.
Reagents:
- Synthetic triglycerides of defined structure (e.g., MLCT vs. physical mixtures)
- Simulated gastric fluid (SGF) and simulated intestinal fluid (SIF)
- Pancreatic lipase and colipase
- Bile salt extracts
- pH monitoring and adjustment systems
Procedure:
- Gastric Phase: Incubate triglyceride samples (0.5-1g) with SGF (pH 2.0) containing pepsin for 30-60 minutes at 37°C with continuous agitation.
- Intestinal Phase: Adjust pH to 6.5-7.0, add SIF containing pancreatic lipase (100-200 U/mL), colipase, and bile salts (10-20 mM).
- Monitoring: Maintain pH at 7.0 using NaOH titration system and record consumption over 60-120 minutes.
- Sampling: Collect aliquots at predetermined timepoints for free fatty acid analysis.
- Analysis: Quantify FFA release via titration or chromatographic methods; calculate bioaccessibility of specific fatty acids using ultracentrifugation to isolate micellar fraction.
Key Parameters:
- Total FFA release (%) after 60-120 minutes
- First-order rate constant (s⁻¹) for FFA release
- Bioaccessibility of specific fatty acids (e.g., DHA, EPA) calculated as percentage in micellar fraction
This methodology enables direct comparison of different triglyceride structures and their behavior under standardized digestive conditions [56].
Gene Expression Profiling
Transcriptomic analysis following MCT administration provides insights into the molecular mechanisms underlying their therapeutic effects. The following protocol adapted from Sanderson et al. [55] details a comprehensive approach:
Objective: To examine the effects of individual dietary fatty acids on hepatic gene expression and identify PPARα-dependent regulation.
Experimental Design:
- Animal Model: Wild-type and PPARα⁻/⁻ mice, fasted for 4 hours
- Intervention: Single oral dose (400μL) of synthetic triglycerides composed of single fatty acids (C18:1, C18:2, C18:3, C20:5, C22:6) or PPARα agonists (WY14643, fenofibrate)
- Tissue Collection: Harvest livers 6 hours post-administration
- RNA Isolation: Extract total RNA using standardized methods
- Microarray Analysis: Hybridize to whole genome arrays (e.g., Affymetrix Mouse Genome 430 2.0 Arrays)
- Data Analysis: Identify significantly changed genes; perform pathway enrichment analysis
Validation: Confirm key findings using quantitative RT-PCR and functional assays relevant to identified pathways.
This approach demonstrated that approximately 98% of genes regulated by unsaturated fatty acids were PPARα-dependent, highlighting the central role of this nuclear receptor in mediating MCT effects [55].
Figure 2: Experimental Workflow for MCT Mechanism Studies - This diagram outlines a comprehensive approach to investigating the molecular mechanisms of MCT action, incorporating genetic models (PPARα knockout) and transcriptomic analysis to identify key pathways.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents for MCT Investigations
| Reagent/Category | Specific Examples | Research Application | Technical Considerations |
|---|---|---|---|
| Defined Triglycerides | Synthetic MLCT; Single-FA TGs (C8, C10, C18:1, C18:2, C22:6) | Controlled structure-function studies; Digestibility assays | Purity critical; Characterization via mass spectrometry |
| In Vitro Digestion System | SGF/SIF; Pancreatic lipase/colipase; Bile salts | Standardized digestibility assessment; Bioaccessibility measurement | pH-stat titration for kinetics; Ultracentrifugation for micellar fraction |
| PPARα Tools | PPARα⁻/⁻ mice; PPARα agonists (WY14643); Reporter assays | Mechanism of action studies; Receptor-specific effects | Confirm genotype; Dose-response for agonists |
| Ketone Analysis | β-hydroxybutyrate assays; ¹¹C-acetoacetate PET imaging | Ketone production and utilization quantification | Temporal considerations for peak levels; Correlation with outcomes |
| Gene Expression Profiling | Microarrays (Affymetrix); RNA-seq; qRT-PCR validation | Comprehensive pathway analysis; Mechanism elucidation | Multiple timepoints; Appropriate statistical correction |
Medium-chain triglycerides represent promising therapeutic agents with applications spanning neurological and metabolic disorders. Their efficacy stems from a combination of unique metabolic properties, including rapid absorption, hepatic metabolism independent of carnitine transport, and potent ketogenic capacity even in the presence of carbohydrates. The structural organization of fatty acids on the triglyceride molecule significantly influences digestive kinetics and bioaccessibility, with important implications for product formulation.
Future research should address several critical questions. First, the long-term metabolic effects of chronic MCT supplementation require further characterization, particularly regarding lipid homeostasis and insulin sensitivity. Second, the relative contributions of ketone-dependent versus ketone-independent mechanisms to cognitive benefits need clarification. Third, individual factors influencing MCT response, including APOE genotype, microbial metabolism, and baseline metabolic status, warrant investigation to enable personalized recommendations.
From a clinical translation perspective, optimization of MCT formulations to balance efficacy with tolerability represents an important challenge. Combining MCTs with other therapeutic modalities may yield synergistic benefits while mitigating potential limitations of individual approaches. As research continues to elucidate the multifaceted mechanisms of MCT action, their potential as therapeutic agents for increasingly diverse applications continues to expand.
Phospholipids, fundamental amphiphilic molecules that constitute cellular membranes, have emerged as critical components in advanced drug delivery systems. This technical review examines the application of phospholipid-based nanocarriers—specifically liposomes and phytosomes—in enhancing the bioavailability and therapeutic efficacy of bioactive compounds. By leveraging their unique structural properties, these systems effectively overcome significant pharmacokinetic limitations associated with poorly soluble drugs, including many herbal phytoconstituents. The content is framed within broader research on dietary lipids, highlighting how the fundamental chemical understanding of triglycerides and phospholipids informs their pharmaceutical application. This review provides drug development professionals with a comprehensive analysis of current methodologies, performance data, and experimental protocols, supported by structured visualizations and practical research tools.
Phospholipids are amphiphilic molecules consisting of a hydrophilic phosphate head group and two hydrophobic fatty acid tails, enabling them to form the fundamental bilayer structure of cellular membranes [61]. In both nutritional and pharmaceutical contexts, this unique structure facilitates the encapsulation and delivery of bioactive compounds. While dietary triglycerides serve primarily as energy sources, phospholipids play more complex structural and functional roles, influencing cellular integrity, membrane fluidity, and molecular transport [23]. The transition from understanding basic lipid structures in nutrition to applying them in drug delivery represents a significant advancement in leveraging biochemical principles for therapeutic benefit.
The chemical structure of phospholipids distinguishes them from dietary triglycerides. Triglycerides comprise a glycerol backbone esterified with three fatty acid chains, functioning mainly as energy storage molecules. In contrast, phospholipids contain a glycerol backbone with two fatty acid chains and a phosphate group attached to a polar head group, creating their characteristic amphiphilic nature [61]. This structural difference underpins their functional divergence: while triglycerides provide metabolic energy, phospholipids form organized structures in aqueous environments, making them ideal for drug delivery applications.
In pharmaceutical sciences, phospholipids have been engineered to overcome significant challenges in drug delivery, particularly for compounds with poor water solubility, low permeability, and consequently limited bioavailability [62] [63]. This review focuses on two primary phospholipid-based delivery systems: liposomes, which are spherical vesicles with aqueous cores surrounded by phospholipid bilayers; and phytosomes, which are complex formations between phytoconstituents and phospholipids that demonstrate superior bioavailability compared to other herbal delivery systems [62].
Phospholipid-Based Drug Delivery Systems
Liposomes: Structure and Mechanisms
Liposomes are spherical vesicles consisting of one or more phospholipid bilayers surrounding aqueous compartments, mimicking biological membranes [61]. Their architecture allows for the encapsulation of both hydrophilic drugs (within the aqueous core) and hydrophobic drugs (within the lipid bilayer), making them versatile carriers for a wide range of therapeutic agents [61]. The fundamental components of liposome formulations typically include biocompatible phospholipids, sphingolipids, cholesterol, and hydrophilic polymers, which collectively determine the system's stability, fluidity, and drug release characteristics [61].
The therapeutic efficacy of liposomal systems stems from several key mechanisms. First, their biocompatible nature reduces immune recognition and clearance, thereby extending systemic circulation time. Second, their ability to encapsulate drugs protects therapeutic compounds from degradation and metabolism before reaching target tissues. Third, the enhanced permeability and retention (EPR) effect, particularly valuable in oncology applications, allows liposomes of specific sizes (typically 10-400 nm) to accumulate preferentially in tumor tissues due to the leaky vasculature and impaired lymphatic drainage characteristic of cancerous growths [61] [64]. This passive targeting mechanism significantly increases drug concentration at the disease site while reducing systemic exposure and associated side effects.
Advanced liposomal systems can be further functionalized through surface modification with targeting ligands such as antibodies, peptides, or small molecules that recognize receptors overexpressed on specific cell types [61]. This active targeting approach enhances the precision of drug delivery, particularly for complex diseases like breast cancer where tumor heterogeneity presents significant treatment challenges [61]. Plant-based phospholipids (PPs) from sustainable sources such as soybeans, sunflower, and canola have gained prominence as alternatives to animal-derived phospholipids due to their lower immunogenic risk, ethical sourcing, cost-effective scalability, and often higher unsaturated fatty acid content which may enhance membrane fluidity and drug encapsulation efficiency [61].
Phytosomes: Advanced Herbal Delivery
Phytosomes represent a technologically advanced delivery system specifically designed to address the bioavailability challenges of herbal phytoconstituents. These complexes are formed through molecular interactions between standardized plant extracts and phospholipids, primarily phosphatidylcholine, in aprotic solvents [62]. The resulting structure differs significantly from liposomes; rather than encapsulating compounds within vesicles, phytosomes form an integral molecular complex where the phytoactive constituents become part of the membrane structure itself [62].
This complexation yields several pharmaceutical advantages. Phytosomes demonstrate significantly higher bioavailability compared to conventional herbal extracts because the phospholipid complex facilitates easier passage through the intestinal barrier and enhances absorption [62]. The phytosomal structure also provides improved protection of active compounds from degradation by digestive secretions and gut microbiota. Additionally, phytosomes show superior pharmacokinetic profiles and tissue distribution efficiency compared to other delivery systems like liposomes, along with enhanced stability and drug loading capacity [62].
Therapeutic applications of phytosomal systems span multiple disease areas, including cancer, osteoarthritis, metabolic syndrome, inflammatory conditions, neurological disorders, and liver diseases [62]. For instance, ginsenoside Rg3, an active component of Panax ginseng with significant anti-tumor, anti-inflammatory, and antioxidant properties, has been successfully formulated into phytosomal systems to overcome its inherent poor water solubility and low permeability, which otherwise restrict clinical application [63].
Table 1: Comparative Analysis of Phospholipid-Based Drug Delivery Systems
| Characteristic | Liposomes | Phytosomes |
|---|---|---|
| Structural Configuration | Concentric phospholipid bilayers surrounding aqueous compartments | Integral molecular complex between phytoconstituents and phospholipids |
| Drug Encapsulation | Hydrophilic drugs in aqueous core, hydrophobic drugs in lipid bilayer | Phytoconstituents hydrogen-bonded to phospholipid polar heads |
| Bioavailability Enhancement | Protection from degradation, EPR effect, extended circulation | Improved intestinal absorption, protection from metabolism |
| Preparation Complexity | Moderate to high, requiring specialized equipment | Relatively simpler, based on complexation in aprotic solvents |
| Drug Loading Capacity | Limited by inner volume and bilayer capacity | Typically higher due to molecular complexation |
| Stability Profile | Potential for oxidation, fusion, drug leakage | Enhanced physical and chemical stability |
| Therapeutic Applications | Oncology, infectious diseases, gene delivery | Herbal medicine, nutraceuticals, chronic diseases |
Quantitative Performance Data
Rigorous evaluation of phospholipid-based delivery systems has generated substantial quantitative data demonstrating their enhanced performance characteristics. The following table summarizes key metrics from preclinical and clinical studies, providing researchers with benchmark data for system evaluation and development.
Table 2: Quantitative Performance Metrics of Phospholipid-Based Delivery Systems
| Performance Parameter | Conventional Formulations | Phospholipid-Based Systems | Experimental Model |
|---|---|---|---|
| Bioavailability Enhancement | Baseline | 3-8 fold increase for phytosomes [62] | Preclinical pharmacokinetic studies |
| Tumor Accumulation | Minimal passive targeting | 2-5 fold increase via EPR effect [61] | Breast cancer xenograft models |
| Systemic Circulation Half-life | Hours | Extended to days in long-acting formulations [65] | Rodent pharmacokinetic studies |
| Drug Solubility | Limited aqueous solubility | Significant improvement for hydrophobic compounds [63] | In vitro solubility assays |
| Cellular Uptake | Variable, concentration-dependent | Enhanced and targeted delivery [61] | Cell culture studies |
| Stability Profile | Standard degradation kinetics | Improved protection from metabolic degradation [62] | Stability testing protocols |
The data clearly demonstrate the substantial advantages of phospholipid-based systems across multiple performance parameters. The significant bioavailability improvements are particularly noteworthy for herbal phytoconstituents, which often exhibit promising in vitro pharmacological activity but poor in vivo performance due to absorption limitations [62]. Similarly, the enhanced tumor accumulation through the EPR effect represents a critical advancement in oncology therapeutics, enabling higher localized drug concentrations while minimizing systemic exposure [61].
Experimental Protocols and Methodologies
Phytosome Preparation Protocol
The preparation of phytosomal complexes follows a standardized methodology that can be adapted for various phytoconstituents. The following protocol details the thin-film hydration method, one of the most widely employed techniques for phytosome production [62]:
Materials Preparation:
- Active phytoconstituent (standardized extract)
- Phospholipids (soy phosphatidylcholine recommended for initial trials)
- Anhydrous aprotic solvent (dioxane, acetone, or ethyl acetate)
- Rotary evaporator with vacuum system
- Hydration buffer (appropriate to final application)
- Probe sonicator or high-pressure homogenizer
Complex Formation:
- Dissolve the phytoconstituent and phospholipid (typically in 1:1 to 1:3 molar ratios) in the selected aprotic solvent in a round-bottom flask.
- Maintain reaction temperature at 40-60°C with continuous stirring for 1-3 hours under reflux conditions to prevent solvent evaporation.
- Monitor reaction completion using TLC or HPLC to confirm complex formation.
Solvent Removal:
- Transfer the reaction mixture to a rotary evaporator and carefully remove the organic solvent under reduced pressure at controlled temperature (not exceeding 40°C).
- Continue until a thin film forms on the inner surface of the flask.
Hydration and Size Reduction:
- Hydrate the dried film with an appropriate buffer (phosphate buffer, pH 6.8, recommended for oral formulations) at a temperature above the phase transition temperature of the phospholipids.
- Rotate the flask continuously for approximately 30-45 minutes until all material is dispersed.
- Subject the resulting suspension to probe sonication (5-10 cycles of 30 seconds pulse with 30 seconds rest) or high-pressure homogenization (5-15 cycles at 500-1500 bar) to achieve desired particle size (typically 50-200 nm).
Purification and Characterization:
- Centrifuge the preparation at low speed (1000 × g for 5 minutes) to remove any uncomplexed material or large aggregates.
- Characterize the final product for particle size distribution (dynamic light scattering), morphology (transmission electron microscopy), complexation efficiency (spectroscopic methods), and drug content (HPLC).
This methodology produces phytosomal complexes with typically higher bioavailability, stability, and drug loading capacity compared to conventional herbal extracts [62]. The critical parameters requiring optimization for each new phytoconstituent include the drug-to-phospholipid ratio, reaction temperature and duration, and hydration conditions.
Liposome Functionalization for Targeted Delivery
Surface functionalization of liposomes enables active targeting to specific tissues or cell types, significantly enhancing therapeutic precision. The following protocol details the post-insertion technique for ligand conjugation to pre-formed liposomes [61]:
Materials:
- Pre-formed liposomes (prepared by any standard method)
- Targeting ligand (antibody fragment, peptide, or small molecule)
- Functionalized phospholipid (DSPE-PEG2000-Maleimide for thiol conjugation)
- Coupling buffer (e.g., HEPES, pH 6.5-7.4)
- Purification system (dialysis membrane or size exclusion chromatography)
- Characterization equipment (DLS, HPLC with size exclusion column)
Ligand Preparation:
- If necessary, introduce reactive functional groups (thiol groups for maleimide chemistry) to the targeting ligand.
- Purify modified ligands using desalting columns or dialysis to remove excess modifying agents.
Liposome Preparation:
- Incorporate 0.5-5 mol% of functionalized phospholipid into the standard liposome formulation during preparation.
- Ensure the reactive group (e.g., maleimide) remains accessible on the liposome surface, typically at the distal end of PEG chains.
Conjugation Reaction:
- Incubate functionalized liposomes with the targeting ligand in coupling buffer for 4-16 hours at 4-25°C with gentle agitation.
- Maintain appropriate ligand excess (typically 2-5 fold molar excess relative to available reactive sites on liposomes) to drive conjugation efficiency.
Purification and Validation:
- Remove unconjugated ligands and reaction byproducts using size exclusion chromatography or tangential flow filtration.
- Characterize the functionalized liposomes for ligand density (spectroscopic methods or specialized assays), size distribution, surface charge, and binding specificity (cell-based assays if appropriate).
This functionalization approach significantly enhances the targeting capability of liposomal systems, particularly valuable in oncology applications where receptor overexpression on cancer cells can be exploited for selective drug delivery [61]. The optimal ligand density must be determined empirically for each application, balancing targeting efficiency against potential immunogenicity or altered pharmacokinetics.
Visualization of Key Concepts
Phospholipid Molecular Structure and Self-Assembly
Phospholipid Assembly Pathways - This diagram illustrates the molecular structure of phospholipids and their self-organization into various drug delivery architectures based on their amphiphilic properties and environmental conditions.
Bioavailability Enhancement Mechanism
Bioavailability Enhancement Mechanism - This workflow diagrams how phospholipid-based systems address the key pharmaceutical limitations of conventional drug forms through multiple complementary mechanisms.
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful development and evaluation of phospholipid-based drug delivery systems requires specific reagents, materials, and instrumentation. The following table details essential components for research in this field.
Table 3: Essential Research Reagents and Materials for Phospholipid Drug Delivery Systems
| Category | Specific Examples | Research Function | Notes for Selection |
|---|---|---|---|
| Phospholipids | Soy phosphatidylcholine, Hydrogenated phospholipids, DSPC, DPPC | Formulation backbone, membrane formation | Purity (>90%) critical for reproducibility; plant sources preferred for reduced immunogenicity [61] |
| Sterol Additives | Cholesterol, Phytosterols | Membrane stability and fluidity modulation | Typically 10-45 mol% of lipid content; affects drug release kinetics |
| Functionalized Lipids | DSPE-PEG2000, Maleimide-PEG-DSPE, Biotinylated lipids | Surface modification, stealth properties, targeting | PEG length (1000-5000 Da) affects circulation half-life [61] |
| Characterization Reagents | Fluorescent probes (DiI, DiO), Calcein, ANTS/DPX assay kits | Membrane integrity, encapsulation efficiency, stability assessment | Fluorescent quenching assays provide sensitive leakage measurement |
| Solvent Systems | Chloroform, Methanol, Ethanol, Aprotic solvents (dioxane) | Lipid dissolution, film formation, complexation | HPLC grade for reproducibility; residue limits critical for in vivo applications |
| Purification Materials | Sephadex G-50, Dialysis membranes (MWCO 10-100 kDa), Tangential flow filters | Removal of unencapsulated drugs, free ligands, solvent residues | Method selection depends on liposome size and stability |
| Cell Culture Models | Caco-2 cells, MCF-7 breast cancer cells, HUVEC cells | Permeability assessment, cytotoxicity, targeting validation | Multiple cell lines recommended for mechanism studies |
| Analytical Standards | Cholesterol standards, Phospholipid calibration kits, Drug reference standards | HPLC/LC-MS quantification, method validation | Certified reference materials essential for regulatory submissions |
This toolkit represents the fundamental requirements for establishing a research program in phospholipid-based drug delivery systems. Additional specialized reagents may be required for specific applications, such as radiolabeled lipids for detailed biodistribution studies or atomic force microscopy supplies for advanced structural characterization.
Phospholipid-based drug delivery systems represent a sophisticated convergence of pharmaceutical technology and fundamental lipid biochemistry. Liposomes and phytosomes have demonstrated significant capabilities in enhancing the bioavailability and therapeutic efficacy of challenging drug candidates, particularly those with poor solubility and permeability characteristics. The continued advancement of these systems leverages growing understanding of lipid membrane behavior, molecular interactions, and disease pathophysiology.
Future developments in this field will likely focus on several key areas: the creation of "smart" systems with externally controlled release profiles using stimuli-responsive materials [65]; increased personalization through targeted delivery approaches; further exploration of sustainable plant-based phospholipid sources [61]; and integration with emerging therapeutic modalities including gene editing tools and cellular therapies. The successful translation of these advanced delivery systems will depend on multidisciplinary collaboration across pharmaceutical sciences, materials engineering, molecular biology, and clinical medicine.
As research continues to elucidate the complex relationships between lipid structures and their biological functions, phospholipid-based delivery systems will play an increasingly important role in overcoming pharmaceutical challenges and realizing the full therapeutic potential of both conventional and novel bioactive compounds.
Phospholipids are amphipathic molecules consisting of a hydrophilic head group containing a phosphate group and two hydrophobic fatty acyl tails [20] [66]. This unique chemical structure enables them to spontaneously form organized interfaces and bilayers in aqueous environments, making them fundamental to biological membranes and essential for emulsion stabilization [67]. In the context of dietary lipids, phospholipids differ structurally from triglycerides—while triglycerides contain three fatty acids esterified to a glycerol backbone, phospholipids replace one fatty acid with a phosphate-containing polar head group [68] [67]. This critical structural difference confers emulsifying properties that are exploited across food and pharmaceutical sciences.
The amphipathic nature of phospholipids drives their spontaneous orientation at oil-water interfaces, with the hydrophilic head group facing the aqueous phase and hydrophobic tails extending into the oil phase [66]. This molecular arrangement reduces interfacial tension and creates physicochemically stable barriers that prevent droplet coalescence [69] [70]. Understanding this structure-function relationship is essential for leveraging phospholipids in complex emulsion systems for food processing and drug delivery applications.
Mechanisms of Emulsion Stabilization
Interfacial Film Formation and Stabilization
Phospholipids stabilize emulsions through several interconnected mechanisms, primarily through the formation of a continuous, viscoelastic film at the oil-water interface that provides a physical barrier against droplet coalescence [69]. The effectiveness of this interfacial film is governed by phospholipid composition, head group characteristics, and fatty acyl chain properties [70].
Table 1: Factors Influencing Phospholipid Emulsion Stabilization Effectiveness
| Factor | Impact on Emulsion Stability | Molecular Basis |
|---|---|---|
| Head Group Size/Charge | Smaller headgroups (PE) allow tighter packing; charge affects electrostatic repulsion | Reduced electrostatic repulsion at lower pH increases interfacial stiffness and attraction between adjacent PLs [70] |
| Fatty Acyl Chain Saturation | Saturated straight chains enable crystalline domain formation; unsaturated chains with bends hinder tight packing | Saturated PLs form interfacial networks through chain crystallization during cooling; unsaturated PLs act as placeholders with weaker PL-PL interactions [70] |
| Interfacial Rheology | Stiffer interfaces resist mechanical deformation and droplet coalescence | Attractive PL-PL interactions increase dilatational and shear moduli, enhancing resistance to interfacial stress [70] |
| Phase Transition Temperature | Determines interfacial behavior under temperature fluctuations | Saturated phospholipids with higher Tm form crystalline domains during cooling, increasing elasticity [70] |
The continuous phase viscosity also significantly impacts emulsion stability, with higher viscosity slowing down droplet movement and reducing creaming or sedimentation rates [69]. Biopolymers such as proteins and polysaccharides can synergistically enhance phospholipid-stabilized emulsions by increasing continuous phase viscosity or forming composite interfacial layers [69].
Comparative Stabilization Mechanisms
Different phospholipid classes exhibit distinct stabilization behaviors based on their molecular structures. Phosphatidylcholine (PC) with its larger, bulkier head group creates different interfacial packing geometries compared to phosphatidylethanolamine (PE) which has a smaller head group [70]. Saturated phospholipids like DSPC (1,2-dioctadecanoyl-sn-glycero-3-phosphocholine) form highly ordered, crystalline interfacial films upon cooling, while unsaturated analogs like DOPC (1,2-di-(9Z-octadecenoyl)-sn-glycero-3-phosphocholine) maintain fluid interfaces due to kinks in their fatty acyl chains [70].
Figure 1: Relationship between phospholipid structure, interfacial properties, and emulsion stabilization mechanisms
Food Science Applications: Fat Globule Stabilization
Dairy Processing and Infant Formula
In dairy applications, phospholipids play crucial roles in stabilizing milk fat globules through natural milk fat globule membranes (MFGM) [71]. The manufacturing processes for infant formula typically disrupt the native phospholipid-coated fat globules, negatively impacting physical stability and nutritional properties [71]. Recent research has demonstrated that adding exogenous phospholipids can compensate for this processing damage and restore fat globule integrity.
A 2025 study systematically investigated how soybean phospholipids (SPs) and egg yolk phospholipids (EYPs) affect the stability and fat globule structure of processed milk through pasteurization, homogenization, and spray drying [71]. The experimental protocol involved:
- Preparation of fortified milk: SPs or EYPs were added to raw milk at 5 mg/mL concentration and dispersed at 6000 rpm for 3 minutes using a high-speed shear disperser
- Pasteurization: Heating at 63°C for 30 minutes to produce pasteurized milk
- Homogenization: Pre-heating to 65°C followed by high-pressure homogenization at 20 MPa pressure
- Spray drying: Converting homogenized milk to powder form
- Analysis: Measuring stability constants, particle size distribution, and interfacial film structure
Table 2: Impact of Phospholipid Addition on Processed Milk Properties [71]
| Sample | Particle Size (nm) | Stability Constant | Fat Globule Structure |
|---|---|---|---|
| Pasteurized Milk (PM) | - | 0.42 | - |
| SP-fortified PM | - | 0.37 | Improved stability |
| EYP-fortified PM | - | 0.37 | Improved stability |
| Homogenized Milk (HM) | 953.39 | 0.30 | Protein-coated globules |
| SP-fortified HM | - | 0.30 | Intermediate improvement |
| EYP-fortified HM | 870.35 | 0.28 | Denser phospholipid film, more intact structure |
The study revealed that EYPs were superior to SPs in restructuring fat globules and enhancing stability in homogenized milk, attributed to their formation of a denser phospholipid interface film [71]. In spray-dried milk powder, EYP supplementation produced particles with more uniform distribution and smoother surfaces, indicating better preservation of fat globule integrity through processing stages [71].
Emulsion Stability in Food Products
Beyond dairy, phospholipids serve as essential emulsifiers in numerous food products including mayonnaise, salad dressings, margarine, and spreads [72] [69]. They prevent destabilization phenomena such as coalescence (merging of droplets), flocculation (clustering of droplets), Ostwald ripening (growth of larger droplets at the expense of smaller ones), and phase separation [69].
The effectiveness of phospholipids in food emulsions depends on their ability to create mechanical barriers against droplet aggregation while modulating interfacial rheology [70]. Lysophospholipids, with their higher hydrophilic-lipophilic balance (HLB), are particularly effective for water-oil emulsions like margarine [20]. Phospholipids also synergize with other food components like proteins and polysaccharides to form composite interfaces that provide enhanced protection against environmental stresses such as pH changes, thermal processing, and mechanical shear [69].
Pharmaceutical Applications
Drug Delivery Systems
Phospholipids have been widely utilized to prepare liposomal, ethosomal, and other nanoformulations of topical, oral, and parenteral drugs [20]. These applications leverage the ability of phospholipids to form supramolecular structures that improve drug bioavailability, reduce toxicity, and enhance permeability across biological membranes [20].
Liposomal formulations encapsulate drugs within phospholipid bilayers, improving targeted delivery and reducing side effects—an application particularly relevant in cancer treatments and vaccine delivery [72]. Ethosomal formulations using phospholipids, such as ketoconazole for transdermal delivery in fungal infections, represent promising drug delivery platforms [20]. The molecular structure of phospholipids enables them to self-assemble into various delivery vehicles with tunable properties based on head group chemistry, fatty acyl chain length, and saturation degree.
Enhanced Bioavailability and Targeting
Phospholipid-based drug delivery systems enhance the bioavailability of active pharmaceutical ingredients through several mechanisms. They increase solubilization of lipophilic drugs, protect susceptible drugs from degradation, and promote cellular uptake through membrane fusion or endocytosis [72]. Pharmaceutical-grade phospholipids with defined compositions (such as DMPC, DPPC, DSPC) enable precise engineering of delivery system characteristics including release kinetics, surface charge, and tissue targeting [20].
Phytosome technology represents a advanced application where phospholipids form molecular complexes with plant-derived active ingredients, significantly enhancing their absorption and bioavailability [68]. Clinical studies demonstrate that such phospholipid complexes can achieve up to 54 times greater cell permeability and 20 times higher plasma levels compared to non-complexed compounds [68].
Analytical Methods and Experimental Protocols
Phospholipid Characterization Techniques
Accurate analysis of phospholipids presents challenges due to the close polarity range between different species [20] [68]. Modern analytical approaches include:
- 31P-NMR Spectroscopy: Provides absolute quantification of phospholipid classes based on phosphorus detection [20]
- HPLC-ELSD (Evaporative Light Scattering Detection): Enables relative quantification with stable baseline and sensitivity independent of double bonds [68]
- HPLC-UV Detection: Utilizes absorption of ester bonds at 205-208 nm, though limited by solvent restrictions [68]
- Thin-Layer Chromatography (TLC): Traditional method for phospholipid separation prior to quantification [68]
Sample preparation typically involves phospholipid purification using solid-phase extraction (SPE) with silica columns to achieve high recovery rates before chromatographic analysis [68]. For fatty acid composition analysis, acid-catalyzed transesterification converts fatty acids in phospholipids to methyl esters followed by GC-MS detection [71].
Interfacial Characterization Protocol
The interfacial properties of phospholipids can be systematically characterized using the following experimental approach based on current research [70]:
- Interfacial Film Formation: Prepare phospholipid solutions in appropriate buffer/organic solvent systems and allow adsorption at oil-water interface
- Dilatational Rheology: Apply oscillating droplet/bubble techniques to measure viscoelastic properties versus deformation frequency
- Interfacial Shear Rheology: Quantify mechanical strength of formed interfacial films under shear deformation
- Interfacial Tension Measurement: Use pendant drop or Wilhelmy plate methods to monitor tension reduction over time
- Temperature Modulation: Implement heating-cooling cycles (e.g., 20°C to 60°C to 5°C) to examine thermal responsiveness
- pH Variation: Assess interfacial behavior across physiological and processing pH ranges (3.0-7.4)
This protocol enables comprehensive characterization of how phospholipid head groups, fatty acyl chains, and environmental conditions influence emulsion stabilization capacity.
Figure 2: Experimental workflow for comprehensive characterization of phospholipid interfacial properties
Research Reagents and Materials
Table 3: Essential Research Reagents for Phospholipid Emulsion Studies
| Reagent/Material | Specifications | Research Application | Functional Role |
|---|---|---|---|
| Soybean Phospholipids (SP) | Commercial grade, purified | Food emulsion studies, infant formula | Natural emulsifier, MFGM substitute [71] |
| Egg Yolk Phospholipids (EYP) | >80% phosphatidylcholine | Comparative stabilization studies | Superior fat globule restructuring [71] |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine | Pharmaceutical liposomes | High transition temperature, stable bilayers [20] [70] |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine | Model membrane studies | Defined phase transition at 41°C [70] |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine | Fluid phase systems | Unsaturated chains, fluid interfaces [70] |
| DSPE | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine | Interfacial packing studies | Small headgroup, tight packing [70] |
| DOPE | 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine | Fusion-promoting systems | Unsaturated PE, hexagonal phase propensity [70] |
| Rd-DOPE | Fluorescently labeled DOPE | Microscopy tracking | Membrane fusion and trafficking studies [71] |
| Cholesterol | Pharmaceutical grade | Membrane fluidity modulation | Increases packing density, regulates fluidity [35] |
Phospholipids serve as versatile emulsifiers and stabilizers in both food and pharmaceutical sciences due to their unique amphipathic molecular structure. Their ability to form ordered interfacial films with tunable rheological properties makes them indispensable for creating stable emulsions, protecting fat globules during processing, and enhancing drug delivery efficiency. The structural characteristics of phospholipids—including head group size/charge, fatty acyl chain length, and saturation degree—critically determine their functionality in emulsion systems.
Future research directions include developing plant-based phospholipid sources to meet clean-label trends, designing precision phospholipids for targeted drug delivery, and leveraging computational approaches to predict phospholipid behavior in complex matrices. As analytical techniques advance, particularly in lipidomics and interfacial characterization, our understanding of structure-function relationships will enable more sophisticated applications of these essential biomolecules across food and pharmaceutical sciences.
The chemical structure of dietary triglycerides and phospholipids is a critical determinant of their physiological behavior and functional outcomes in both food and pharmaceutical systems. Tailoring the fatty acid (FA) composition of these lipids allows researchers to precisely modulate their nutritional value, physical properties, and metabolic fate [73] [74]. This strategic modification aligns with a broader thesis in lipid research: that structure dictates function. Advances in metabolic engineering and process optimization now enable the production of lipids with customized compositions, moving beyond naturally occurring profiles to create specialized ingredients for targeted applications in functional foods and drug development [75] [74].
This technical guide synthesizes current methodologies for structurally modifying lipids, providing researchers with experimental protocols, data analysis, and visualization tools to advance work in this field.
Microbial Tailoring of Fatty Acids
Oleaginous yeasts represent powerful microbial platforms for sustainable lipid production, capable of accumulating oils from various carbon sources. Their lipid accumulation is induced under nutrient-limiting conditions, with nitrogen limitation being the most effective strategy [75].
Optimizing Fermentation Parameters
Response Surface Methodology (RSM) provides a systematic approach for identifying optimal production conditions. The synergistic effect of carbon-to-nitrogen (C/N) ratio and cultivation temperature significantly impacts both lipid yield and fatty acid profile in oleaginous yeasts [75].
Table 1: Optimal Fermentation Conditions for Oleaginous Yeasts [75]
| Yeast Strain | Optimal Temperature | Optimal C/N Ratio | Resulting Lipid Accumulation | Key Fatty Acids Produced |
|---|---|---|---|---|
| Cutaneotrichosporon oleaginosus | 30 °C | 175 g/g | 71% increase vs. average conditions | Palmitic (C16:0), Stearic (C18:0), Oleic (C18:1), Linoleic (C18:2) |
| Yarrowia lipolytica | 21 °C | 140 g/g | 66% increase vs. average conditions | Palmitic (C16:0), Stearic (C18:0), Oleic (C18:1), Linoleic (C18:2) |
Steering Fatty Acid Composition
Temperature variation provides a powerful lever for steering fatty acid saturation levels. Lower cultivation temperatures shift production toward more unsaturated oils, increasing unsaturation by 14% in C. oleaginosus and 31% in Y. lipolytica. Conversely, higher temperatures promote the production of longer-chain saturated fatty acids [75].
Metabolic Engineering in Plants
Metabolic engineering in oilseed crops enables the production of "designer" oils with fatty acid profiles tailored for specific nutritional and industrial applications. This approach has successfully produced omega-3 long-chain polyunsaturated fatty acids (LC-PUFAs) at levels comparable to native marine organisms [74].
Key Metabolic Engineering Strategies
Transgenic Expression of Heterologous Enzymes: Seed-specific expression of biosynthetic activities from other species can introduce novel fatty acids or alter existing profiles. The expression of a 3-ketoacyl-CoA synthase (KCS) gene from Lunaria annua in transgenic plants and yeast successfully increased nervonic acid content [74].
Manipulation of the Kennedy Pathway: The acyl-CoA-dependent activities of this pathway are crucial for triacylglycerol (TAG) assembly. Modifying enzymes like phosphatidylcholine:diacylglycerol cholinephosphotransferase (ROD1) can channel unsaturated fatty acids into storage oil [74].
Engineering Omega-3 LC-PUFA Biosynthesis: Recent progress includes engineering the synthesis and accumulation of omega-3 long-chain polyunsaturated fatty acids in seed oils of transgenic plants. This involves introducing and optimizing multiple desaturase and elongase enzymes to convert endogenous fatty acids into these valuable compounds [74].
Table 2: Metabolic Engineering Targets for Tailored Plant Oils [74]
| Engineering Target | Key Enzymes/Genes | Functional Outcome | Application Relevance |
|---|---|---|---|
| Omega-3 LC-PUFA Accumulation | Δ6-desaturase, Δ5-desaturase, Elongases | Production of EPA and DHA in seeds | Human nutrition, pharmaceutical |
| Very Long Chain Fatty Acids (VLCFAs) | Ketoacyl-CoA Synthase (KCS) | Increased nervonic acid content | Industrial lubricants, medical use |
| Oleic Acid Content | Desaturase suppression, Thioesterase expression | High-oleic, low-palmitic acid oils | Improved oil stability, biodiesel |
| Hydroxy Fatty Acids | Fatty Acid Hydroxylases (e.g., C12 hydroxylase) | Production of ricinoleic acid | Industrial chemicals, polymers |
Controlled Lipid Digestion for Functional Outcomes
The targeted modification of triglyceride structure directly influences lipid digestion and absorption rates in the human gastrointestinal tract, enabling the development of functional and personalized foods [73].
Factors Regulating Lipid Digestion
Fatty Acid Chain Length and Saturation: Shorter-chain and unsaturated fatty acids are generally digested and absorbed more rapidly than longer-chain and saturated ones. Modifying the FA profile of dietary triglycerides can therefore control their assimilation rate [73].
Triglyceride Structure: The positional distribution of fatty acids on the glycerol backbone (sn-1, sn-2, sn-3) affects susceptibility to pancreatic lipase, which primarily hydrolyzes the sn-1 and sn-3 positions [73].
Emulsion Properties and Interface Composition: The physicochemical characteristics of lipid emulsions, including droplet size and interfacial composition, significantly impact the rate and extent of lipid digestion [73].
Strategies for Modulating Lipid Assimilation
Emulsion-Based Approaches: Designing oil-in-water (O/W) emulsions with specific interfaces (e.g., using layer-by-layer (LbL) techniques, whey protein isolate (WPI), or polysaccharides) can control lipase accessibility and digestion kinetics [73].
Structured Lipids: Creating specific triglyceride structures (e.g., through interesterification) that are less accessible to digestive enzymes can reduce fat absorption, particularly beneficial for weight management [73].
Bioactive Components: Incorporating lipase inhibitors such as epigallocatechin gallate (EGCG) from green tea can temporarily reduce fat assimilation, offering another mechanism for controlled lipid delivery [73].
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Reagents for Lipid Modification Studies
| Reagent/Category | Function/Application | Specific Examples |
|---|---|---|
| Oleaginous Yeast Strains | Microbial platforms for oil production | Cutaneotrichosporon oleaginosus ATCC 20509, Yarrowia lipolytica DSM 1345 [75] |
| Defined Media Components | Controlled cultivation conditions | Glycerol (carbon source), Urea (nitrogen source), KH₂PO₄, NaH₂PO₄·7H₂O, MgSO₄·7H₂O [75] |
| Metabolic Engineering Tools | Genetic modification of oilseed crops | KCS (Ketoacyl-CoA Synthase) genes, Δ6-desaturase, ROD1 phosphatidylcholine:diacylglycerol cholinephosphotransferase [74] |
| Lipase Enzymes | In vitro digestion studies | Human Pancreatic Lipase (HPL), Human Gastric Lipase (HGL), Pancreatic Phospholipase A2 (PLA2) [73] |
| Emulsifiers & Stabilizers | Interface engineering for controlled digestion | Whey Protein Isolate (WPI), Citric Acid Esters (CITREM), Diacetyl Tartaric Acid Esters (DATEM), Propylene Glycol Alginate (PGA) [73] |
| Lipase Inhibitors | Modulating lipid digestion rates | Epigallocatechin Gallate (EGCG) [73] |
Overcoming Stability and Metabolic Challenges in Lipid-Based Formulations
Triglyceride (TAG) polymorphism, the ability of a single chemical compound to crystallize in multiple distinct solid forms, is a fundamental property that governs the physical and functional characteristics of fats. This phenomenon has been documented as early as 1853, when Duffy observed multiple melting points in tristearin [76]. The polymorphic behavior of triglycerides directly determines critical performance parameters in food, pharmaceutical, and energy storage applications, including melting profile, stability, texture, and drug release kinetics. The molecular architecture of triglycerides—consisting of a glycerol backbone esterified to three fatty acids of varying chain lengths, saturation patterns, and positional distribution—creates a system capable of diverse molecular packing arrangements in the solid state [8] [76].
Within the context of dietary triglycerides and phospholipids research, controlling polymorphism transitions from a scientific curiosity to an essential technological requirement. The crystallization pathways and end-state polymorphic forms significantly impact the bioavailability of lipid-based drug delivery systems, the mouthfeel and shelf-stability of confectionery fats, and the efficiency of phase-change materials in thermal energy storage [76]. This technical guide provides a comprehensive framework for understanding, characterizing, and controlling triglyceride polymorphism to achieve desired stability and melting profiles, with specific methodologies tailored for research and industrial applications.
Fundamental Aspects of Polymorphic Forms
Characterization of Primary Polymorphs
Triglycerides predominantly crystallize into three basic polymorphic forms, traditionally labeled in order of increasing stability and melting temperature as alpha (α), beta prime (β'), and beta (β). Each form exhibits distinctive structural features and physical properties, as detailed in Table 1 [76].
Table 1: Characteristics of Primary Triglyceride Polymorphs
| Polymorph | Subcell Packing | Melting Temperature | Structural Characteristics | Stability | Typical Morphology |
|---|---|---|---|---|---|
| Alpha (α) | Hexagonal | Lowest | Molecules randomly rotated around chain axis; loose packing | Metastable, kinetically favorable | Amorphous mass of small crystals |
| Beta Prime (β') | Orthorhombic | Intermediate | Spherulitic crystals; moderately ordered | Metastable | Small needles or spherulites |
| Beta (β) | Triclinic | Highest | Molecules locked in definite position; chains tilted ~65°; tight packing | Thermodynamically stable | Large, needle-like crystals |
The alpha (α) form represents the least ordered arrangement, where molecules are organized with random degrees of rotation around the chain axis but at constant distances, corresponding to hexagonal subcells [76]. This polymorph is thermodynamically unstable but kinetically favorable, making it prevalent under rapid cooling conditions. The beta prime (β') form typically consists of spherulitic crystals with orthorhombic subcell packing and exhibits melting points intermediate between the α and β forms [76]. This polymorph is highly influenced by impurities, with concentrations exceeding 2-3% potentially completely blocking its formation. The beta (β) form represents the most thermodynamically stable configuration with triclinic subcell packing, characterized by molecules locked in definite positions with hydrocarbon chains tilted at approximately 65° to the end-group planes [76].
Polymorphic Transition Pathways
The transformation between polymorphic states follows monotropic behavior, meaning transitions occur irreversibly from less stable to more stable forms. Figure 1 illustrates the characteristic transition pathways and relationships between different polymorphic forms.
Figure 1: Polymorphic Transition Pathways in Triglycerides
As illustrated in Figure 1, the α form crystallizes directly from the melt under fast cooling conditions and can reversibly melt back to the liquid state. However, in the solid state, polymorphic transformations proceed irreversibly from α to β' to β, or directly from α to β, with the complete transition to the most stable β form often requiring extended time periods and specific thermal conditions [76]. The β' form is particularly noteworthy as it occurs more frequently and demonstrates enhanced stability in the presence of unsaturation or asymmetrical molecular structures [76]. Recent research has highlighted that triglycerides maintain a thermal "memory" of past crystalline forms; once formed, β crystals will not revert to less stable phases during thermal cycling unless the thermal history is erased via high-temperature treatments [76].
Advanced Characterization Techniques
X-ray Scattering Methodologies
X-ray scattering techniques form the cornerstone of polymorphic characterization, providing nanoscale structural information about triglyceride crystals. Small-angle X-ray scattering (SAXS) reveals lamellar stacking patterns at length scales corresponding to the molecular length of triglycerides (typically 0.05 < q < 7 nm⁻¹), while wide-angle X-ray scattering (WAXS) provides information about the polymorphic state through subcell packing details (7 < q < 20 nm⁻¹) [8]. More recently, ultra-small angle X-ray scattering (USAXS) has emerged as a powerful technique for determining TAG crystallite aggregation on larger length scales [8].
The analysis of X-ray scattering data enables several critical determinations for polymorphism control:
Electron Density Profile (EDP) Calculations: EDP decomposes the lamellar repeat distance into bilayer and monolayer contributions, providing insight into molecular organization [8].
Chain Tilt Angle Determination: Refined estimations of the hydrocarbon chain tilt angle within the bilayer region, typically approximately 65° for the β form [8] [76].
Crystallite Size Analysis: Application of the Scherrer equation to diffraction peak broadening enables determination of crystal domain sizes [8].
Area Per Hydrocarbon Chain: Calculation of the cross-sectional area occupied by each hydrocarbon chain within the crystal lattice [8].
Table 2: X-ray Scacing Parameters for Polymorph Identification
| Analysis Technique | Structural Parameter | Alpha (α) | Beta Prime (β') | Beta (β) |
|---|---|---|---|---|
| SAXS/Long Spacing | Lamellar Repeat Distance (Å) | ~48-52 (SSS) | ~41-46 (SSS) | ~45-49 (SSS) |
| WAXS/Short Spacing | Primary Reflection (Å) | ~4.2 (broad) | ~4.2 and ~3.8 (strong) | ~4.6, 3.9, 3.7 (strong) |
| Subcell Packing | Crystal System | Hexagonal | Orthorhombic | Triclinic |
| Chain Tilt | Angle to Basal Plane | ~90° | ~70-75° | ~60-65° |
Complementary Analytical Methods
Differential scanning calorimetry (DSC) provides complementary thermodynamic data, including melting points, enthalpies of fusion, and polymorphism transition temperatures. The thermal properties of triglycerides are directly influenced by their chemical structure, with melting points and enthalpies increasing with chain length and decreasing with unsaturation [76].
Stimulated Raman scattering (SRS) microscopy has emerged as a powerful label-free technique for visualizing drug distribution and crystalline forms in pharmaceutical formulations [77]. This advanced vibrational spectroscopy method provides significant capability in pharmaceutical development by enabling determination of intracellular drug localization and metabolism without requiring fluorescent labeling [77]. SRS microscopy can be particularly valuable for tracking polymorphic transformations in complex matrices and during processing.
Experimental Protocols for Polymorphism Control
Thermal Processing and Tempering Protocols
Thermal history represents the most fundamental parameter controlling polymorphic outcomes. The following standardized tempering protocols can be employed to target specific polymorphic forms:
Alpha (α) Form Crystallization:
- Heat triglyceride sample to at least 15°C above its complete melting point to erase thermal history.
- Rapidly cool (quench) the melt to 10-15°C below the α form melting point at a rate exceeding 5°C/min.
- Hold at this temperature for characterization or immediately analyze, as the α form will quickly begin transforming to more stable forms.
Beta Prime (β') Form Crystallization:
- Melt the sample as above and cool rapidly to a temperature just above the α melting point.
- Hold at this temperature for 30-60 minutes, allowing for nucleation and growth of the β' form.
- Alternatively, seed the melt with pre-formed β' crystals and maintain at a temperature where β' is the stable form.
Beta (β) Form Crystallization:
- Employ slow, controlled cooling from the melt (0.5-1°C/min) to a temperature just above the β melting point.
- Maintain at this temperature for extended periods (several hours to days).
- Seeding with stable β crystals significantly accelerates this process.
- Solvent crystallization from appropriate organic solvents can also produce the β form directly.
Compositional Engineering Strategies
Molecular structure fundamentally influences polymorphic behavior, with the following compositional factors serving as powerful control parameters:
Chain Length Disparity: Asymmetric triglycerides (e.g., Sat-2OSat, where the chain on sn-1 position is two carbons shorter) and those with chain length differences preferentially stabilize the β' form [8].
Unsaturation Introduction: Mono-unsaturated triglycerides (e.g., SatOSat) demonstrate strong β'-stabilizing tendencies and can form molecular compounds with specific mixing behavior [8].
Additive Incorporation: Specific additives, including partial glycerides, phospholipids, and specialized emulsifiers, can inhibit or promote specific polymorphic transformations through surface adsorption and crystal habit modification.
Tailored Mixtures: Binary and ternary triglyceride mixtures with carefully selected components can be designed to maintain desirable β' forms through incompatible chain lengths that impede transformation to the β form.
Industrial Applications and Stability Management
Pharmaceutical Formulation Strategies
In pharmaceutical development, polymorphic control is critical for consistent drug delivery and stability. Lipid-based excipient systems such as LipoGalen, a family of polyglycerol esters, have been specifically engineered to crystallize in a single stable modification without polymorphism, ensuring consistent drug release profiles and processing behavior [78]. These functional lipid-based excipients find application in rectal and vaginal dosage forms, controlled-release tablets, hot melt extrusion for bioavailability enhancement, taste masking via hot melt coating, and lipid nanoparticles for advanced delivery systems [78].
The monotropic polymorphism of triglycerides has been innovatively applied in the development of nanoparticulate tracer systems to monitor temperature exposure during pharmaceutical processing. Using tristearin and tripalmitin with distinct melting temperatures, researchers have created tracer systems that accurately record the maximum temperatures experienced by lipid nanoparticles during high-energy processes like spray drying and dual centrifugation [79] [80]. This approach exploits the irreversible nature of polymorphic transitions to provide a thermal history record that is otherwise difficult to obtain.
Food Product Applications
In food systems, polymorphic control directly influences functional properties. Cocoa butter and cocoa butter equivalents exemplify systems where specific polymorphic forms (Form V, a β form) must be achieved through precise tempering to obtain proper snap, gloss, and heat resistance [8]. In contrast, margarines and spreads often rely on β'-crystals for optimal texture and spreadability, maintained through formulation with β'-stabilizing triglycerides and emulsifiers.
Butter and anhydrous milk fat represent complex multi-triglyceride systems where fractionation techniques can separate low-melting and high-melting fractions with distinct polymorphic behaviors [81]. Low-melting point fractions of buffalo milk fat (LMPF) obtained through dry fractionation at 10°C, 15°C, and 25°C demonstrate increased unsaturated fatty acid content and altered triglyceride composition (increased C44-C54 triglycerides) compared to the parent milk fat [81]. These fractions exhibit different crystallization behaviors and polymorphic stability, expanding potential industrial applications.
Thermal Energy Storage Systems
Triglycerides represent promising phase-change materials (PCMs) for latent heat storage applications due to their high enthalpy of fusion, bio-based origin, and tunable melting points through fatty acid profile manipulation [76]. For effective implementation in thermal energy storage, polymorphism must be controlled to ensure consistent and predictable phase-change transitions during repeated thermal cycling [76]. The thermal "memory effect" in triglycerides, where materials retain knowledge of previous crystalline forms, can be advantageous in these applications once the desired polymorph is achieved [76].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagent Solutions for Polymorphism Studies
| Reagent/Material | Function in Polymorphism Research | Application Examples |
|---|---|---|
| Pure Monoacid TAGs (e.g., Tristearin, Tripalmitin) | Reference compounds for fundamental polymorphism studies | Establishing baseline structural data for homologous series [8] |
| Mixed-Acid TAGs (e.g., OPO, POP) | Model compounds for investigating molecular compound formation | Studying chain length and saturation effects on polymorphic stability [8] |
| Stabilizers (e.g., Poloxamers, Polysorbates) | Surface-active agents that influence crystal growth and modification | Screening for effects on triglyceride modifications in nanoparticle systems [80] |
| LipoGalen Excipients | Non-polymorphic lipid bases for controlled drug delivery | Formulation of stable dosage forms with consistent release profiles [78] |
| Deuterated Solvents | Media for solvent-mediated crystallization and polymorphism studies | Investigating solvent effects on polymorphic outcome [76] |
The controlled manipulation of triglyceride polymorphism represents an essential capability at the intersection of materials science, pharmaceutical technology, and food engineering. Through systematic application of the characterization methodologies, experimental protocols, and formulation strategies outlined in this technical guide, researchers can precisely engineer stability and melting profiles to meet specific application requirements. The continuing advancement of analytical techniques, particularly in X-ray scattering and vibrational spectroscopy, provides increasingly sophisticated tools for elucidating the complex structure-function relationships that govern polymorphic behavior in triglyceride systems. As research progresses, the deliberate design of triglyceride compositions and processing protocols to target specific polymorphic outcomes will enable enhanced performance across diverse technological domains, from optimized drug delivery systems to sustainable phase-change materials and premium food products.
Phospholipids (PLs) are amphiphilic molecules that serve as fundamental structural and functional components in biological membranes and engineered emulsion systems. Their unique molecular geometry, featuring a phosphate-containing polar head-group and non-polar hydrocarbon chains, enables spontaneous assembly and stabilization at oil-water interfaces [82]. In the context of dietary lipids research, the chemical structure of PLs and triglycerides profoundly influences their metabolic fate and biological activity [83]. This technical guide examines the comparative structural and functional properties of soy and egg yolk phospholipids, providing evidence-based strategies for their selection and interfacial engineering in pharmaceutical and food applications.
The critical role of phospholipids extends beyond emulsion stabilization to include modulation of digestive processes and bioaccessibility. Recent research has demonstrated that triglyceride structure significantly impacts digestibility and bioaccessibility of nutritional lipids during in vitro simulated digestion [83]. These findings underscore the importance of molecular structure in designing emulsion-based delivery systems for pharmaceutical applications.
Compositional Analysis: Soy vs. Egg Yolk Phospholipids
Molecular Diversity and Structural Characteristics
Soy and egg yolk phospholipids differ significantly in their molecular composition, which directly influences their interfacial behavior and functional properties in emulsions.
Table 1: Comparative Composition of Soy and Egg Yolk Phospholipids
| Component | Soy Lecithin | Egg Yolk Lecithin | Functional Significance |
|---|---|---|---|
| Phosphatidylcholine (PC) | 20-24% | 68-72% | Primary emulsifier; reduces interfacial tension |
| Phosphatidylethanolamine (PE) | 21-25% | 15-20% | Promotes hexagonal phases; affects curvature |
| Phosphatidylinositol (PI) | 17-21% | 0.5-1.5% | Binding properties; charge modulation |
| Phosphatidylserine (PS) | 2-4% | 1-2% | Charge characteristics; biological activity |
| Lysophospholipids | Variable | Increased after PLA2 treatment | Enhanced emulsification; faster adsorption |
| Fatty Acid Profile | Higher unsaturated (C18:2) | Balanced saturated/unsaturated | Membrane fluidity; oxidative stability |
Egg yolk lecithin contains a significantly higher proportion of phosphatidylcholine (PC), the phospholipid most effective at stabilizing oil-in-water interfaces [84]. The fatty acid profile of egg yolk phospholipids tends to be more balanced between saturated and unsaturated chains compared to soy phospholipids, which contain higher amounts of polyunsaturated fatty acids, particularly linoleic acid [85]. This compositional difference affects both the interfacial behavior and oxidative stability of the resulting emulsions.
Functional Performance Metrics
Table 2: Quantitative Performance Comparison of Soy vs. Egg Yolk Phospholipids in Emulsion Systems
| Performance Parameter | Soy Phospholipids | Egg Yolk Phospholipids | Enzymatically Modified Egg Yolk |
|---|---|---|---|
| Interfacial Tension Reduction | Moderate | High | Very High |
| Adsorption Kinetics | Moderate | Fast | Very Fast |
| Creaming Stability | Variable | High | 95.13% improvement after treatment [86] |
| Particle Size (after treatment) | - | 82.99 nm (U+P) [86] | - |
| Interfacial Protein Load | - | High at high ionic strength | Higher at low ionic strength [87] |
| Solubility Improvement | - | 27.90% after U+P treatment [86] | - |
Egg yolk phospholipids demonstrate superior interfacial activity in their native state, while soy phospholipids can be effectively modified to enhance functionality. Enzymatic modification with phospholipase A2 (PLA2) converts phospholipids to lysophospholipids, which exhibit faster adsorption kinetics and improved emulsification properties [87]. The compositional advantages of egg yolk phospholipids make them particularly valuable for pharmaceutical applications where purity and performance are critical.
Interfacial Engineering Strategies
Molecular Modification Techniques
Enzymatic Modification
Enzymatic treatment with phospholipase A2 (PLA2) hydrolyzes the acyl group at the sn-2 position of triglycerides, converting phospholipids to lysophospholipids and free fatty acids [87]. This modification significantly alters the interfacial properties:
- Accelerated Adsorption: Lysophospholipids adsorb more rapidly at oil-water interfaces
- Reduced Interfacial Tension: Modified egg yolk achieves lower equilibrium tension
- Enhanced Dispersibility: Lysophospholipids improve solubility and dispersion stability
- Thinner Films: Formed films are thinner and drain more rapidly
The conversion to lysophospholipids transforms the interfacial behavior from protein-dominated to surfactant-dominated systems, with faster adsorption kinetics and altered viscoelastic properties [87].
Ultrasound-Assisted pH-Shifting Treatment
Combined physical and chemical modification strategies can substantially enhance functionality:
Figure 1: Workflow of Combined Ultrasound-pH Shifting Treatment for Egg Yolk Proteins
This synergistic treatment applies extreme pH conditions to induce protein unfolding, followed by ultrasound cavitation that generates localized microenvironments of high temperature and pressure, disrupting non-covalent bonds between proteins [86]. The sequential application (P → U) demonstrates particularly pronounced effects:
- Structural Disruption: Disrupts insoluble egg yolk granules facilitating interfacial adsorption
- Particle Size Reduction: Reduces average particle size from 160.97 nm to 82.99 nm
- Enhanced Electrostatic Repulsion: Increases surface charge of egg yolk proteins
- Interfacial Reinforcement: Significantly enhances interfacial pressure and viscoelasticity
Interfacial Characterization and Analysis
The interfacial behavior of phospholipids can be quantitatively characterized through several advanced techniques:
Langmuir Trough Measurements: Surface pressure (π) - molecular area (A) isotherms provide information on phase transitions and molecular packing [82]. Phospholipids exhibit characteristic isotherms with distinct liquid-expanded (LE) to liquid-condensed (LC) phase transitions upon compression.
Oscillatory Barrier Rheology: Determines the dilatational viscoelasticity of phospholipid monolayers through repeated compressions and expansions while recording deviations in surface pressure [84]. The dilatational modulus E is composed of elastic (E') and viscous (E") components.
Interfacial Stress Rheometry: Measures monolayer response to shear deformation using an oscillatory force applied to a magnetic needle at the air/water interface [84]. This technique reveals the solid-like or fluid-like characteristics of the interfacial film.
Surface Potential Measurements: Utilizes a vibrating capacitor method with electrodes positioned above the water surface and immersed in the subphase to determine molecular density and dipole moment at the interface [84].
Experimental Protocols
Protocol 1: Ultrasound-Assisted pH-Shifting of Egg Yolk
Objective: Enhance emulsification and interfacial properties of egg yolk protein through combined physical and chemical treatment [86].
Materials:
- Fresh egg yolk
- Phosphate-buffered saline (PBS, 10 mM, pH 7.0)
- HCl and NaOH solutions for pH adjustment
- Ultrasonication equipment (e.g., probe-type sonicator)
Methodology:
- Egg Yolk Solution Preparation: Separate egg yolk from fresh eggs, remove excess egg whites. Dilute with PBS to 1% protein concentration.
- pH-Shifting Treatment: Adjust pH to extreme alkaline (pH 12.0) or acidic (pH 2.0) conditions using HCl/NaOH. Maintain under constant stirring for 1 hour.
- Neutralization: Return solution to pH 7.0.
- Ultrasound Treatment: Apply ultrasonication at controlled power and duration (typically 20 kHz, 400 W for 15-30 min).
- Control Treatments: Include individual treatments (U-only, P-only) and sequential combinations (P→U, U→P) for comparison.
Key Analysis:
- Solubility: Determine by centrifugation and Biuret method
- Particle Size: Dynamic light scattering
- Interfacial Pressure: Langmuir trough or drop shape analyzer
- Emulsion Stability: Creaming index after storage
Protocol 2: Enzymatic Modification of Egg Yolk with PLA2
Objective: Improve emulsification properties through enzymatic conversion of phospholipids to lysophospholipids [87].
Materials:
- Native egg yolk
- Phospholipase A2 enzyme
- Buffer solutions (appropriate for enzyme activity)
- Substrate and reagents for chemical analysis
Methodology:
- Egg Yolk Preparation: Separate and dilute egg yolk to appropriate concentration.
- Enzymatic Reaction: Add PLA2 at optimal concentration (typically 0.5-2.0% w/w). Incubate at optimal temperature and pH with constant agitation.
- Enzyme Inactivation: Heat treatment to denature enzyme after reaction completion.
- Product Characterization: Analyze lysophospholipid content, interfacial tension, and emulsion properties.
Key Analysis:
- Chemical Composition: TLC or HPLC for phospholipid profiling
- Interfacial Tension: Pendant drop tensiometry
- Film Properties: Thin film balance analysis
- Emulsion Characterization: Droplet size, ζ-potential, stability assessment
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagent Solutions for Phospholipid Emulsion Studies
| Reagent/Category | Specific Examples | Function/Application |
|---|---|---|
| Phospholipid Sources | Soy lecithin, Egg yolk lecithin, Hydrogenated lecithins | Primary emulsifiers; interfacial stabilization |
| Modification Enzymes | Phospholipase A2, Lipases | Structural modification; enhanced functionality |
| Analytical Standards | Phosphatidylcholine, Phosphatidylethanolamine, Lysophospholipids | Quantification; method calibration |
| Interfacial Characterization | Langmuir trough, Drop shape analyzer, Microfluidic devices | Interfacial tension; rheological properties |
| Particle Analysis | Dynamic light scattering, Zeta potential analyzer, Static light scattering | Droplet size; stability assessment |
| Emulsion Stabilizers | Xanthan gum, Modified cellulose, Proteins (whey, soy) | Viscosity modification; enhanced stability |
Applications and Performance Optimization
Pharmaceutical Formulation Strategies
Phospholipid-based emulsions serve as delivery vehicles for lipophilic bioactive compounds, with performance dictated by source selection and interfacial engineering:
- Liposomal Encapsulation: Egg yolk phospholipids with high PC content form more stable, uniform liposomes for drug delivery [84]
- Bioavailability Enhancement: Engineered interfaces improve solubilization and absorption of poorly water-soluble drugs
- Structured Lipid Systems: Medium- and long-chain triglycerides (MLCT) demonstrate modified digestibility profiles and enhanced bioaccessibility of bioactive fatty acids [83]
Stability Optimization Strategies
Figure 2: Multi-faceted Approach to Emulsion Stability Optimization
Emulsion stability is governed by multiple interdependent factors including interfacial composition, continuous phase viscosity, and droplet characteristics [69]. Combined approaches addressing multiple destabilization mechanisms simultaneously yield the most robust systems:
- Interfacial Engineering: Ultrasound-assisted pH-shifting and enzymatic modification create reinforced interfaces with enhanced viscoelasticity [86]
- Continuous Phase Control: Biopolymers like xanthan gum form three-dimensional network structures that inhibit droplet migration [69]
- Droplet Architecture: Pickering stabilization with solid particles provides mechanical barriers to coalescence [69]
Source selection between soy and egg yolk phospholipids represents a critical decision point in emulsion design, with egg yolk providing superior inherent functionality while soy offers economic advantages and modification potential. Interfacial engineering through enzymatic modification and ultrasound-assisted pH-shifting enables significant enhancement of natural phospholipid performance.
Future research directions should focus on:
- Precision modification techniques for targeted molecular structures
- Multi-scale characterization of interface dynamics during digestion
- Sustainable sourcing of high-performance phospholipids
- Integration of computational modeling with experimental validation
The optimization of phospholipid-based emulsions bridges fundamental research on chemical structure of dietary lipids with practical applications in pharmaceutical and functional food development, offering enhanced delivery systems for bioactive compounds with improved stability and bioavailability.
In the context of research on the chemical structure of dietary lipids, the milk fat globule membrane (MFGM) represents a unique and biologically significant architecture. In its native state, the fat in human and bovine milk exists as an oil-in-water emulsion, where a triglyceride core is enveloped by a tri-layer MFGM, rich in phospholipids, sphingolipids, cholesterol, and glycoproteins [88] [89]. This intricate structure is not merely a physical barrier; it is a functional interface critical for fat digestion, nutrient absorption, and immunological protection [88] [90]. The phospholipids, being amphipathic, form the fundamental structural skeleton of this membrane, stabilizing the globule within the aqueous milk environment and facilitating critical biological interactions [89].
However, industrial processing—essential for the safety and shelf-life of dairy products like infant formula—systematically dismantles this native architecture. Key unit operations, including heat treatment, homogenization, and spray drying, induce severe physical and chemical disruptions. Heat treatment exceeding 60°C alters the physical state of phospholipids, causing their displacement from the MFGM into the serum phase and destroying the lipid domain structure [71]. Homogenization, while reducing fat globule size to enhance physical stability, violently strips away the native MFGM. It replaces the phospholipid-rich membrane with a primarily casein and whey protein-based interface, creating a globule structure that is fundamentally alien to biological systems [71] [90]. Subsequently, spray drying subjects these already-altered globules to further stress, affecting their physical, chemical, and microstructural characteristics [71]. The cumulative effect is a loss of bioactive MFGM components, disintegration of the original fat globule structure, and the formation of an inferior, processed globule with compromised nutritional and functional properties [71]. This structural degradation can hinder lipid digestion, alter metabolic responses, and negatively influence the developing gut microbiota [91] [90]. Consequently, strategies to mitigate this processing-induced damage are a critical frontier in nutritional science and dairy technology.
Scientific Rationale: The Case for Exogenous Phospholipids
The rationale for using exogenous phospholipids is grounded in their fundamental role as surface-active, amphipathic molecules capable of spontaneously reorganizing at oil-water interfaces. The core hypothesis is that the introduction of exogenous phospholipids during processing can competitively displace or co-adsorb with milk proteins on the newly formed fat globule surface, thereby creating a bio-mimetic membrane that more closely resembles the native MFGM.
The efficacy of this approach is influenced by the biochemical composition of the phospholipid source. Key properties include:
- Fatty Acid Saturation: Phospholipids with higher saturation levels, such as those found in egg yolk, contribute to greater membrane stability and a denser interfacial film. This is attributed to tighter molecular packing and higher phase transition temperatures compared to their more unsaturated counterparts [71] [90].
- Polar Head Group and Sphingolipid Content: The specific profile of phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), and sphingomyelin (SM) determines the interfacial behavior, charge, and biological activity of the reconstituted membrane. Sphingomyelin, in particular, forms a rigid, ordered domain (lipid rafts) with cholesterol, which enhances structural integrity [89].
- Source Material: Soybean phospholipids (SPs) and egg yolk phospholipids (EYPs) are the most commonly studied sources. EYPs are often characterized by a higher proportion of saturated fatty acids and a distinct phospholipid profile, which can translate to superior performance in restructuring fat globules and enhancing the stability of dairy emulsions during and after processing [71].
Beyond their emulsifying and structural roles, phospholipids are bioactive entities. They serve as precursors for signaling molecules, influence gut barrier function, and exhibit anti-inflammatory and antimicrobial properties [88] [89]. Therefore, their restoration is not only a matter of physical structure but also of biological functionality, directly aligning with the goals of advanced nutritional and pharmaceutical development.
Experimental Evidence and Quantitative Data
Recent research provides compelling quantitative evidence supporting the efficacy of exogenous phospholipids in mitigating processing damage. A pivotal study directly compared the effects of adding soybean phospholipids (SPs) or egg yolk phospholipids (EYPs) to milk subjected to a series of standard processing steps [71].
The table below summarizes key quantitative findings from this study, demonstrating the differential effects of SP and EYP supplementation on the physical stability and structural properties of processed milk.
Table 1: Impact of Exogenous Phospholipids on Processed Milk Properties [71]
| Processing Stage | Sample | Key Physical Stability Metric | Key Structural Metric | Observation |
|---|---|---|---|---|
| Pasteurization | Phospholipid-free milk | Stability constant: 0.42 | N/A | Baseline for comparison |
| Milk + SPs or EYPs | Stability constant: 0.37 | N/A | Both phospholipids improved stability vs. control; no significant difference between SP and EYP. | |
| Homogenization | Milk + SPs | Stability constant: 0.30; Particle size: 953.39 nm | Interface film structure | Good improvement over non-fortified milk. |
| Milk + EYPs | Stability constant: 0.28; Particle size: 870.35 nm | Denser phospholipid interface film | EYPs superior to SPs, resulting in smaller globules and a more stable, intact membrane. | |
| Spray Drying | Milk Powder + EYPs | N/A | More uniform distribution and smoother surface of powder particles | The improved homogenized globule structure led to superior final powder quality. |
The findings clearly indicate that EYPs were superior to SPs in restructuring fat globules and enhancing the stability of newly formed fat globules, particularly during the disruptive homogenization step [71]. This superior performance is attributed to the formation of a denser phospholipid interface film, which leads to a more intact fat globule structure. This structural advantage is then carried through to the final spray-dried product, where milk powder particles supplemented with EYPs showed a more uniform distribution and smoother surface [71].
Methodological Protocols: Key Experimental Approaches
To implement and validate the use of exogenous phospholipids, robust and reproducible methodologies are essential. The following protocols detail the core procedures for preparing phospholipid-fortified milk and analyzing the resulting fat globule structure.
Preparation of Phospholipid-Fortified Processed Milk
This protocol is adapted from established methods for evaluating phospholipids in dairy processing [71].
1. Materials and Reagents:
- Raw milk.
- Phospholipid source (e.g., purified Soybean Phospholipids (SPs) or Egg Yolk Phospholipids (EYPs)).
- High-speed shear disperser (e.g., IKA T25).
- High-pressure homogenizer (e.g., ATS Engineering AMH-3).
- Spray dryer.
2. Phospholipid Purification: Crude phospholipid materials are purified based on their insolubility in acetone. Dissolve 2g of phospholipid material in 10mL of acetone and stir magnetically for 30 minutes. Remove the acetone by filtration and dry the residual filter residue under a stream of nitrogen gas. The dried sample is the purified phospholipid for use [71].
3. Preparation of Fortified Pasteurized Milk: a. Add SPs or EYPs to raw milk at a target concentration (e.g., 5 mg/mL). b. Disperse the mixture at 6000 rpm for 3 minutes using a high-speed shear disperser to ensure initial incorporation. c. Pasteurize the milk by heating for 30 minutes at 63°C. The resulting samples are designated as SP-PM and EYP-PM [71].
4. Preparation of Fortified Homogenized Milk: a. Pre-heat the pasteurized milk samples (PM, SP-PM, EYP-PM) to 65°C. b. Homogenize the pre-heated milk using a high-pressure homogenizer at a defined pressure (e.g., 20 MPa). The resulting samples are designated as HM, SP-HM, and EYP-HM [71].
5. Preparation of Spray-Dried Milk Powder: Subject the homogenized milk samples (HM, SP-HM, EYP-HM) to standard spray-drying conditions to produce the final milk powder.
Analytical Techniques for Fat Globule Characterization
Particle Size Analysis: The particle size distribution of homogenized milk samples is typically measured using dynamic light scattering (e.g., with a Malvern Zetasizer). This quantifies the effectiveness of homogenization and phospholipid coating in achieving a fine, stable emulsion [71] [90].
Stability Constant Measurement: The physical stability of the emulsion can be quantified using analytical centrifugation or by measuring the stability constant via light scattering techniques, where a lower value indicates a more stable emulsion system [71].
Phospholipid Interface Film Characterization: The structure and density of the interfacial film can be investigated using confocal laser scanning microscopy (CLSM). Fluorescent dyes specific for phospholipids (e.g., Rd-DOPE) are used to visualize the membrane architecture directly, allowing for qualitative assessment of film integrity and density [71].
Phospholipid Profiling via HPLC-ELSD: The phospholipid composition of raw materials and final products can be determined using High-Performance Liquid Chromatography coupled with an Evaporative Light Scattering Detector (HPLC-ELSD). a. Lipid Extraction: Total lipids are extracted using a chloroform-methanol mixture (e.g., 2:1 v/v) via a method based on Folch et al. [92]. b. SPE Purification: Phospholipids are separated from neutral lipids using Solid-Phase Extraction (SPE) on a silica cartridge. Neutral lipids are eluted with non-polar solvents (e.g., hexane:diethyl ether), while phospholipids are recovered with polar solvents (e.g., methanol) [92]. c. HPLC-ELSD Analysis: The purified PL fraction is analyzed on a normal-phase silica column. A gradient elution is applied, and the separated PL classes (PC, PE, PI, PS, SM) are detected by ELSD. Identification is made by comparing retention times to known standards [92].
Pathways and Workflow: From Damage to Restoration
The following diagram synthesizes the core problem, the intervention strategy, and the resulting outcomes into a single, coherent pathway, illustrating the logical flow from processing-induced damage to functional restoration.
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table catalogs key reagents, materials, and instruments essential for conducting research in the field of milk fat globule restoration using exogenous phospholipids.
Table 2: Research Reagent Solutions for MFGM Restoration Studies
| Item | Function / Application | Exemplary Details / Rationale |
|---|---|---|
| Soybean Phospholipids (SPs) | A common, plant-based source of phospholipids for interfacial studies and emulsion stabilization. | Used as a benchmark against which to compare other phospholipid sources. Requires purification to remove neutral lipids [71]. |
| Egg Yolk Phospholipids (EYPs) | An animal-based phospholipid source often demonstrating superior stabilization and biomimetic properties. | Contains a higher proportion of saturated fatty acids and a distinct PL profile, contributing to denser, more stable interfacial films [71]. |
| Rd-DOPE Fluorescent Dye (e.g., Lissamine Rhodamine B-labeled DOPE) | A vital reagent for visualizing the phospholipid membrane via Confocal Laser Scanning Microscopy (CLSM). | Incorporates into the phospholipid layer, allowing direct visualization of the interface structure and integrity [71]. |
| HPLC-ELSD System with Normal-Phase Column | The core analytical platform for separating and quantifying individual phospholipid classes (PC, PE, PI, PS, SM). | Provides a robust method for profiling the phospholipid composition of both raw materials and final dairy products [92]. |
| High-Pressure Homogenizer | Instrument for creating fine, stable emulsions and simulating industrial processing conditions. | Essential for preparing experimental milk samples and studying the protective effect of phospholipids under high-shear stress [71]. |
| Milk Fat Globule Membrane (MFGM) Ingredients | Complex dairy-derived ingredients used as a positive control or a multi-component intervention. | Commercially available ingredients (e.g., Lacprodan MFGM-10) containing a mix of MFGM proteins and polar lipids, used to study holistic MFGM benefits [93]. |
Implications for Product Development and Future Research
The strategic application of exogenous phospholipids extends beyond academic interest, offering tangible pathways for innovating dairy-based nutritional products, particularly infant formula. The evidence indicates that formulas containing large, phospholipid-coated lipid droplets can modulate the infant gut microbiota, enriching beneficial bacteria (e.g., Rominococcaceae, Lachnospiraceae) while reducing opportunistic pathogens (e.g., Klebsiella, Streptococcus) [90]. This microbial profile is more akin to that of breast-fed infants. Furthermore, such interventions have been shown to attenuate excessive weight gain and adiposity in infancy, a metabolic programming effect that may reduce long-term obesity risk [90].
Future research should focus on several key areas:
- Source Optimization: Systematic comparison of phospholipids from novel sources (e.g., milk-derived PLs from buttermilk or whey) for their functional efficacy and bioactivity [92].
- Synergistic Formulations: Exploring the combination of phospholipids with other bioactive compounds, such as docosahexaenoic acid (DHA) and lutein, utilizing the MFGM as a natural delivery system to enhance the stability and bioavailability of these sensitive nutrients [93].
- Clinical Endpoints: Conducting long-term clinical trials to validate the sustained benefits of phospholipid-restored formulas on cognitive development, immune function, and metabolic health.
In conclusion, the restoration of milk fat globule structure through exogenous phospholipids represents a paradigm shift from simply replicating the gross composition of human milk to mimicking its sophisticated supramolecular structure. This approach holds significant promise for bridging the functional gap between conventional dairy products and the golden standard of human milk, with profound implications for infant nutrition and beyond.
The challenge of poor solubility presents a formidable barrier in the development of effective therapeutics and in the optimization of nutrient absorption. For drug compounds, poor aqueous solubility directly limits bioavailability—the proportion of an administered dose that reaches systemic circulation—often resulting in subtherapeutic concentrations and erratic absorption profiles [94]. This issue is particularly prevalent in pharmaceutical development, where approximately 40% of approved drugs and nearly 90% of drug candidates fall into the category of poorly water-soluble compounds [95]. Similarly, the absorption of hydrophobic nutrients, particularly fat-soluble vitamins and complex lipids, faces analogous challenges within the aqueous environment of the gastrointestinal tract.
The Biopharmaceutics Classification System (BCS) categorizes compounds based on their solubility and permeability characteristics. Most problematic are BCS Class II (low solubility, high permeability) and Class IV (low solubility, low permeability) compounds, which together represent over 80% of new drug candidates [96]. Overcoming these bioavailability hurdles requires sophisticated strategies that address the fundamental physicochemical properties governing dissolution and absorption, many of which can be informed by studying nature's own solutions for handling hydrophobic compounds, particularly in the absorption of dietary lipids.
Foundational Science: Lipid Structures and Physiological Absorption Pathways
Structural Chemistry of Dietary Lipids
Understanding the chemical structure of dietary triglycerides and phospholipids provides critical insights for developing bioavailability enhancement strategies. The fundamental architecture of these lipids defines their behavior in biological systems and offers templates for drug delivery system design.
Triglycerides, the most common lipid in both foods and the human body, consist of a glycerol backbone esterified with three fatty acids of varying chain lengths and saturation patterns [97]. This structure creates a predominantly hydrophobic molecule that serves as the primary form of energy storage in the body. The stereospecific numbering (sn) system identifies the position of each fatty acid on the glycerol backbone as sn-1, sn-2, and sn-3, which influences their metabolic fate during digestion [97].
Phospholipids exhibit a more amphipathic character crucial to their biological function. Their structure replaces one fatty acid with a phosphate group and typically a nitrogen-containing compound such as choline (in phosphatidylcholine) [97]. This arrangement creates a molecule with a hydrophilic head (phosphate and associated groups) and hydrophobic tails (fatty acid chains), enabling them to function as natural emulsifiers and key components of cellular membranes.
Natural Lipid Absorption Mechanisms
The physiological absorption of dietary lipids provides a blueprint for enhancing the bioavailability of poorly soluble compounds. The process involves sophisticated emulsification, enzymatic degradation, and reassembly mechanisms that overcome the inherent hydrophobicity of these nutrients.
The journey begins with emulsification in the duodenum, where bile salts secreted from the gallbladder break down large fat droplets into smaller ones, significantly increasing the surface area for enzymatic action [28]. Pancreatic lipase then hydrolyzes triglycerides at the sn-1 and sn-3 positions, producing free fatty acids and monoacylglycerols [28]. These digestive products combine with bile salts to form mixed micelles—amphipathic aggregates that solubilize hydrophobic compounds in their core and transport them to the intestinal mucosa for absorption [28].
Within the enterocytes, resynthesized triglycerides are incorporated into chylomicrons—triglyceride-rich lipoprotein particles that transport dietary lipids through the lymphatic system, bypassing first-pass metabolism [28]. This natural lymphatic transport pathway is particularly relevant for enhancing the bioavailability of lipophilic drugs, as it can be leveraged to improve systemic exposure.
Table 1: Key Lipid Structures and Their Relevance to Bioavailability
| Lipid Type | Structural Features | Biological Function | Relevance to Bioavailability |
|---|---|---|---|
| Triglycerides | Glycerol + 3 fatty acids | Energy storage, insulation | Model for lipid-based delivery systems |
| Phospholipids | Glycerol + 2 fatty acids + phosphate group | Cell membrane structure, emulsification | Natural emulsifiers for solubilization |
| Mixed Micelles | Bile salts + phospholipids + lipid digestion products | Intestinal lipid transport | Blueprint for synthetic solubilizing systems |
| Chylomicrons | Triglyceride-rich lipoprotein particles | Systemic lipid transport | Model for lymphatic drug transport |
Pharmaceutical Strategies for Bioavailability Enhancement
Lipid-Based Drug Delivery Systems
Lipid-based drug delivery systems (LBDDS) represent one of the most promising approaches for enhancing the bioavailability of poorly soluble drugs. These systems leverage the natural lipid digestion and absorption pathways, effectively mimicking the processing of dietary fats [98]. By encapsulating or solubilizing hydrophobic drugs in lipid excipients, LBDDS can significantly increase solubilization and absorption, resulting in enhanced bioavailability [98].
The spectrum of lipid-based formulations includes nanoemulsions, self-emulsifying drug delivery systems (SEDDS), liposomes, solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs) [95]. Each system offers distinct advantages: nanoemulsions provide large surface areas for dissolution; SEDDS form fine emulsions upon gentle agitation in the GI tract; liposomes encapsulate drugs in phospholipid bilayers; while SLNs and NLCs offer improved stability and controlled release profiles [95]. These systems enhance bioavailability through multiple mechanisms, including prolonging gastrointestinal residence time, stimulating lymphatic transport, and enhancing membrane permeability [98].
Nanotechnology Approaches
Nanotechnology has emerged as a powerful tool for overcoming solubility limitations through particle size reduction and enhanced surface area. Drug nanocrystals represent a carrier-free approach where the drug itself is reduced to nanoscale dimensions, significantly increasing the dissolution velocity and saturation solubility according to the Ostwald-Freundlich and Noyes-Whitney equations [95]. With typical sizes between 200-500 nm, nanocrystals combine high drug loading with industrial scalability and applicability to virtually all poorly soluble drugs [95].
More complex nanocarriers include polymeric nanoparticles, dendrimers, and inorganic carriers such as mesoporous silica nanoparticles (MSNs) and layered double hydroxides (LDHs) [95]. These systems can be engineered for targeted delivery and responsive release behaviors. For instance, pH-sensitive nanoparticles can be designed to protect drugs in the stomach and release them in the intestine, while ligand-functionalized nanoparticles enable active targeting to specific tissues or cells [99] [100].
Molecular and Solid-State Modification Strategies
At the molecular level, several strategies directly modify the drug substance to enhance solubility. Salt formation is particularly effective for ionizable compounds, with hydrochloride and sodium salts being the most common among FDA-approved drugs [95]. For non-ionizable compounds, co-crystal formation with compatible co-formers can significantly improve dissolution rates and bioavailability without covalent modification of the API [95]. The development of amorphous solid dispersions (ASDs) represents another major advancement, where the drug is dispersed in a polymer matrix in its high-energy amorphous state, providing enhanced apparent solubility and dissolution rates compared to crystalline forms [96].
Advanced manufacturing technologies like hot melt extrusion and spray drying have proven particularly valuable for producing ASDs, creating amorphous solid dispersions with excellent success in enhancing bioavailability of poorly soluble compounds [96]. The application of Quality-by-Design (QbD) principles further ensures robust formulation development by systematically understanding the impact of material attributes and process parameters on critical quality attributes [96].
Table 2: Comparison of Bioavailability Enhancement Strategies
| Strategy | Mechanism of Action | Key Advantages | Limitations/Challenges |
|---|---|---|---|
| Lipid-Based Formulations | Enhanced solubilization, lymphatic transport, membrane fluidity | Biocompatibility, leverages natural pathways, wide applicability | Stability issues, limited drug loading, excipient compatibility |
| Nanocrystals | Increased surface area, dissolution velocity | High drug loading, carrier-free, applicable to most PWSDs | Physical stability, potential for Ostwald ripening |
| Amorphous Solid Dispersions | High-energy amorphous state, polymer-based supersaturation | Significant solubility enhancement, wide polymer selection | Physical instability, crystallization risk |
| Salt Formation | Improved dissolution through ionization | Well-established regulatory pathway, significant for ionizables | Limited to ionizable compounds, potential for precipitation |
| Co-crystals | Altered crystal packing, improved dissolution | Applicable to non-ionizables, no covalent modification | Co-former selection complexity, regulatory considerations |
| Cyclodextrin Complexation | Molecular encapsulation in hydrophobic cavity | True molecular solution, protection from degradation | Limited loading capacity, potential for toxicity at high doses |
Experimental Methodologies and Research Toolkit
Key Experimental Protocols
Solubility Enhancement Protocol: Lipid-Based Formulations The development of self-emulsifying drug delivery systems (SEDDS) begins with excipient screening to identify optimal oils, surfactants, and co-surfactants that provide maximum drug solubility [98]. The protocol involves:
- Equilibrium solubility studies in various lipid excipients using shake-flask method at 37°C for 24-72 hours
- Pseudoternary phase diagram construction to identify self-emulsification regions using water titration method
- Formulation optimization via experimental design (e.g., Box-Behnken) evaluating droplet size, emulsification time, and drug loading capacity
- In vitro lipolysis model to simulate intestinal digestion using pancreatin extract and bile salts with pH-stat titration
- Stability assessment under accelerated conditions (40°C/75% RH) for 1-3 months
Nanocrystal Production Protocol: Media Milling The production of drug nanocrystals via wet media milling follows this standardized protocol [95]:
- Preparation of drug suspension in stabilizer solution (e.g., 1-2% w/w HPMC or PVP)
- Loading into milling chamber with milling media (typically 0.3-0.6 mm zirconium oxide beads)
- Milling process at appropriate agitator speed (2000-4000 rpm) for 2-8 hours maintaining temperature <45°C
- Separation of beads using sieve or separation system
- Characterization of particle size (D50, D90), zeta potential, crystalline form (PXRD), and dissolution profile
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Reagents for Bioavailability Enhancement Studies
| Reagent/Category | Function/Application | Examples |
|---|---|---|
| Lipid Excipients | Solubilize drugs, form emulsion systems | Medium-chain triglycerides (Miglyol), oleic acid, soy lecithin, Labrafil, Gelucire |
| Surfactants | Reduce interfacial tension, stabilize emulsions | Polysorbate 80, Cremophor EL, Tween 80, Span 80, Solutol HS-15 |
| Polymers for Amorphous Dispersion | Maintain supersaturation, inhibit crystallization | HPMC, HPMCAS, PVP, PVP-VA, Soluplus, Eudragit |
| Cyclodextrins | Form inclusion complexes | HP-β-CD, SBE-β-CD, γ-cyclodextrin |
| Stabilizers for Nanosuspensions | Prevent aggregation of nanoparticles | Poloxamer 407, Tween 80, PVP K30, HPMC, D-α-tocopheryl PEG succinate |
| Lipolysis Model Components | Simulate intestinal environment | Pancreatin extract, taurocholate salts, phospholipids, calcium chloride |
| Permeability Enhancers | Improve membrane transport | Sodium caprate, medium-chain glycerides, acyl carnitines |
Visualization of Key Pathways and Workflows
Lipid Digestion and Drug Absorption Pathway
Diagram 1: Lipid Digestion and Drug Absorption Pathway
Formulation Strategy Selection Workflow
Diagram 2: Formulation Strategy Selection Workflow
The challenges of poor solubility and limited bioavailability represent a critical frontier in pharmaceutical sciences and nutrient research. The strategies discussed—from lipid-based systems that leverage natural absorption pathways to nanotechnological approaches that manipulate materials at the molecular level—provide a robust toolkit for addressing these challenges. The continued advancement of these technologies, particularly through the integration of Quality-by-Design principles, computational modeling, and precision medicine approaches, promises to further enhance our ability to deliver challenging compounds.
Future directions will likely focus on personalized nanomedicine tailored to individual patient physiology, stimuli-responsive systems that release their payload in response to specific biological triggers, and multifunctional nanocarriers that combine therapeutic and diagnostic capabilities [99] [100]. The ongoing study of natural lipid absorption mechanisms will continue to inspire novel delivery strategies, creating a virtuous cycle between fundamental biological understanding and technological innovation. As these advanced delivery systems mature, they will play an increasingly vital role in translating promising therapeutic candidates into clinically effective medicines and optimizing the bioavailability of essential nutrients.
The chemical structures of dietary triglycerides and phospholipids dictate their profoundly divergent metabolic fates and physiological impacts. Triglycerides, serving as efficient energy reservoirs, and phospholipids, acting as structural and signaling components of membranes, engage in a delicate biological balance. Disruption of this equilibrium has significant metabolic consequences, contributing to pathologies such as hepatosteatosis, insulin resistance, and cardiovascular disease. This whitepaper synthesizes current research to elucidate how the distinct structures of these lipids govern their absorption, trafficking, and functional roles, and explores emerging therapeutic strategies aimed at optimizing this balance for improved metabolic health.
At the core of lipid metabolism lies a fundamental dichotomy originating from chemical structure. Triglycerides (TGs) consist of a glycerol backbone esterified to three fatty acid chains, creating a predominantly hydrophobic molecule designed for dense energy storage in lipid droplets within adipocytes [28] [101]. In contrast, phospholipids (PLs) possess a glycerol backbone with two fatty acid tails and a phosphate-containing polar head group [41]. This amphipathic nature—with hydrophobic tails and a hydrophilic head—renders them ideal for forming the phospholipid bilayer of cellular membranes, creating a selective barrier that controls the passage of substances and facilitates cellular communication [41] [102].
The structural difference, while seemingly minor, dictates solubility, metabolic processing, and ultimate biological function. TGs, being insoluble, require packaging into lipoprotein complexes like chylomicrons and VLDL for transport in aqueous plasma [103] [28]. PLs, by virtue of their polar head groups, can self-assemble into membranes and are integrated into the monolayer surface of lipoproteins, providing structural integrity and determining surface properties [102]. Understanding this structural basis is essential for navigating their metabolic consequences.
Structural Determinants and Metabolic Fates
Triglycerides: Optimized for Energy Storage and Mobilization
The metabolism of triglyceride-rich lipoproteins (TRLs) is a central process in systemic energy homeostasis.
- Synthesis and Assembly: Hepatic TG synthesis begins with the stepwise lipidation of apoB100 via the microsomal triglyceride-transfer protein (MTP) in the endoplasmic reticulum. This process forms nascent pre-VLDL, which can mature into either smaller, triglyceride-poor VLDL2 or larger, triglyceride-rich VLDL1 particles, the latter through the bulk addition of triglycerides from cytoplasmic lipid droplets [103]. In the intestine, dietary fats are incorporated into chylomicrons, which contain apoB48 and transport dietary lipids to peripheral tissues [28].
- Transport and Hydrolysis: TRLs, including VLDL and chylomicrons, are secreted into the circulation where they undergo lipolysis by lipoprotein lipase (LPL) anchored to capillary endothelial cells. This hydrolysis releases free fatty acids (FAs) and glycerol for uptake by adipose tissue for storage or by muscle for β-oxidation and energy production [103] [28].
- The Triglyceride/Fatty Acid (TG/FA) Cycle: Intracellular TGs are not static. Recent studies using multi-labeling strategies with alkyne fatty acid tracers have demonstrated that stored TGs undergo continuous degradation and re-synthesis, a process known as TG cycling. In adipocytes, the estimated half-life of a TG molecule is a remarkably rapid 2-4 hours [104]. This futile cycle consumes energy but provides a critical mechanism for remodeling the stored fatty acid pool, allowing for the modification of released FAs through elongation and desaturation (e.g., conversion of saturated FAs to monounsaturated FAs) [104].
Phospholipids: Architects of Membrane Integrity and Signaling
The composition and organization of phospholipids in the plasma membrane are dynamically regulated and directly influence cellular signaling and metabolic health.
- Membrane Asymmetry and Domain Formation: The plasma membrane exhibits transverse asymmetry: the inner leaflet is enriched in phosphatidylethanolamine (PE) and phosphatidylserine (PS), while the outer leaflet is rich in phosphatidylcholine (PC) and sphingolipids [105] [102]. Laterally, membranes are organized into microdomains, such as lipid rafts—sphingomyelin and cholesterol-rich regions that serve as signaling platforms [102].
- Direct Modulation of Signaling Proteins: Membrane lipid composition directly regulates the activity and orientation of key signaling proteins. For instance, the constituency of anionic phospholipids can modulate the orientation of K-Ras, altering the exposure of its catalytic domain to the cytosol and thus its ability to interact with effectors [105]. Similarly, the saturation level of phospholipid fatty acyl chains can affect membrane fluidity and the signaling efficiency of receptors like GPCRs and Toll-like receptor 4 (TLR4) [105].
- Generation of Bioactive Lipid Mediators: Phospholipids serve as reservoirs for precursor fatty acids. Upon enzymatic cleavage (e.g., by phospholipase A2), these FAs give rise to potent signaling molecules. For example, membrane-derived arachidonic acid is the precursor for eicosanoids (prostaglandins, leukotrienes), while docosahexaenoic acid (DHA) is released and converted to resolvins and protectins, which are potent anti-inflammatory and pro-resolving mediators [45] [106].
Table 1: Key Functional Differences Between Triglycerides and Phospholipids
| Feature | Triglycerides | Phospholipids |
|---|---|---|
| Primary Function | Energy storage, insulation [28] [101] | Membrane structure, signaling, vesicle trafficking [41] [102] |
| Metabolic Pathway | Lipolysis (via ATGL, HSL, LPL), β-oxidation [28] | Remodeling (via PLAs, acyltransferases), precursor hydrolysis [102] [106] |
| Transport Form | Chylomicrons, VLDL, IDL [103] [28] | Component of all lipoprotein surfaces [28] |
| Cellular Location | Lipid droplets [104] | Plasma membrane, organelle membranes [41] [102] |
| Key Bioactive Metabolites | Free Fatty Acids, Glycerol | Diacylglycerol (DAG), Inositol Trisphosphate (IP3), Eicosanoids, Resolvins [105] [106] |
Metabolic Consequences of Imbalance
An imbalance favoring energy storage via triglycerides at the expense of membrane integrity and appropriate signaling can drive metabolic dysfunction.
Hepatic Steatosis and Dyslipidemia
Hepatic accumulation of TGs, or non-alcoholic fatty liver disease (NAFLD), is intrinsically linked to the oversecretion of large, triglyceride-rich VLDL1 particles [103]. When the flux of fatty acids to the liver (from the diet, de novo lipogenesis, or adipose tissue lipolysis) exceeds oxidative and secretory capacity, TGs accumulate. Furthermore, impaired phospholipid metabolism can compromise very-low-density lipoprotein (VLDL) secretion, as phospholipids are essential structural components of the lipoprotein particle [103]. This can exacerbate hepatic lipid accumulation.
Adipose Tissue Dysfunction and Inflammation
White adipose tissue (WAT) is not merely a passive storage depot. Dysfunctional expansion of WAT, characterized by adipocyte hypertrophy and hypoxia, triggers a low-grade inflammatory state [45]. The lipid form in the diet can influence this outcome. Research in murine models has shown that supplementing DHA/EPA as phospholipids, compared to triglycerides, was more effective in reducing adipocyte hypertrophy and low-grade inflammation in WAT. This superior efficacy was correlated with a more potent modulation of the endocannabinoid system and increased production of anti-inflammatory lipids like N-docosahexaenoylethanolamine in WAT [45].
Insulin Resistance and Altered Signaling
Membrane phospholipid composition directly impacts insulin sensitivity. The degree of fatty acid saturation in membrane phospholipids influences membrane fluidity, which can affect the conformation and signaling of insulin receptors [105]. Diets rich in saturated fats can incorporate saturated phospholipids into membranes, potentially reducing fluidity and impairing insulin signal transduction. Conversely, phospholipids containing polyunsaturated fatty acids (PUFAs) like DHA can maintain fluidity and support proper receptor function [106]. Additionally, chronic inflammation driven by dysfunctional adipose tissue releases pro-inflammatory cytokines that activate signaling pathways (e.g., JNK, IKKβ) which interfere with insulin signaling, promoting systemic insulin resistance [45].
Experimental Approaches and Methodologies
Tracing Triglyceride Cycling and Fatty Acid Remodeling
Understanding the dynamic nature of lipid stores requires sophisticated tracing methodologies.
Multi-Labeling Strategy with Alkyne Fatty Acids:
- Objective: To directly demonstrate and quantify intracellular TG/FA cycling and the subsequent remodeling of the stored FA pool [104].
- Protocol:
- Tracer Design: A combination of three alkyne-tagged fatty acids (FA;Ys) is used to mirror dietary FA diversity: an odd-chain medium FA (11:0;Y), an even-chain polyunsaturated FA (18:2;Y), and a stable isotope-labeled saturated long-chain FA (13C9-16:0;Y). This allows for unequivocal MS discrimination of their metabolic products [104].
- Cell Treatment: Differentiated 3T3-L1 adipocytes are incubated with the triple FA;Y mixture (e.g., 50 µM each) for a pulse period (e.g., 1 hour).
- Chase Phase: The medium is replaced with a tracer-free medium, and cells are harvested at multiple time points (e.g., 0, 6, 24, 48 hours).
- Lipid Extraction and Analysis: Cellular lipids are extracted and analyzed via mass spectrometry coupled with click-chemistry-based sample multiplexing.
- Data Processing: Custom software (e.g., LipidXplorer with mfql files) is used to identify and quantify hundreds of labeled lipid species, including single- and multi-labelled TGs. The flux of labels between different lipid pools and the appearance of elongated or desaturated products of the original FA;Ys are tracked over time [104].
- Key Outcome: This protocol provides direct evidence of TG cycling by tracking the incorporation of a defined set of FAs from one TG pool into a new TG pool, allowing for the estimation of TG half-life (2-4 hours in 3T3-L1 adipocytes) and demonstrating that cycling facilitates the modification of stored FAs [104].
Diagram 1: Experimental workflow for tracing triglyceride cycling and fatty acid remodeling using alkyne fatty acid tracers.
Evaluating the Metabolic Efficacy of Phospholipid vs. Triglyceride Forms
Comparative studies are crucial for understanding how lipid structure influences bioavailability and metabolic outcomes.
Prevention/Reversal of Obesity-Associated Disorders in Mice:
- Objective: To determine whether the molecular form of dietary omega-3 PUFA (phospholipid vs. triglyceride) differentially affects the prevention and reversal of high-fat diet-induced metabolic disorders [45].
- Protocol:
- Diets: C57BL/6J mice are fed one of several high-fat diets (cHF, ~35% wt/wt lipids) for 9 weeks (prevention study) or after initial induction of obesity (reversal study).
- Control: Corn oil-based high-fat diet (cHF).
- Experimental: cHF-based diets where corn oil is partially replaced by DHA/EPA, admixed either as triglycerides (cHF+ω3TG) or phospholipids (cHF+ω3PL), with doses typically at 10 or 30 g DHA/EPA per kg diet [45].
- Outcome Measurements:
- Systemic Metabolism: Body weight gain, adiposity, glucose tolerance test (GTT), plasma lipids (triglycerides, NEFA), insulin levels.
- Tissue Analysis: Liver lipid content (hepatosteatosis), adipocyte size, markers of WAT inflammation.
- Molecular Analysis: Bioavailability of DHA/EPA (tissue levels in PLs), endocannabinoid and related lipid mediator profiling in WAT [45].
- Diets: C57BL/6J mice are fed one of several high-fat diets (cHF, ~35% wt/wt lipids) for 9 weeks (prevention study) or after initial induction of obesity (reversal study).
- Key Findings: DHA/EPA administered as phospholipids were superior to triglycerides in preventing glucose intolerance and reducing hepatosteatosis. In the reversal study, only the phospholipid form reduced plasma insulin and adipocyte hypertrophy, correlating with better modulation of the WAT endocannabinoid system [45].
Table 2: Key Reagent Solutions for Lipid Metabolism Research
| Research Reagent / Tool | Function / Application |
|---|---|
| Alkyne Fatty Acids (e.g., 11:0;Y, 13C9-16:0;Y) [104] | Metabolic tracers that enable click-chemistry-based tagging and highly sensitive, specific MS detection of a wide range of labeled lipid species. |
| Stable Isotope-Labeled Lipids (e.g., 2H, 13C) [104] | Conventional tracers for following lipid metabolic fluxes using mass spectrometry. |
| LipidXplorer Software [104] | Open-source software for targeted identification and quantification of lipid species from mass spectrometry data using molecular fragment queries (mfql). |
| Specific Dietary Formulations (e.g., cHF+ω3PL) [45] | Defined diets for precisely testing the metabolic effects of different lipid classes (e.g., phospholipid vs. triglyceride) in animal models. |
| LC-MS/MS Platforms | Essential technology for comprehensive lipidomics, allowing for the identification and quantification of hundreds to thousands of lipid species from complex biological samples. |
Signaling Pathways at the Crossroads of Metabolism and Membrane Biology
Cellular metabolic status is communicated through lipid-mediated signaling pathways that are intimately connected to membrane composition.
Diagram 2: Key lipid-influenced signaling pathways in metabolic regulation and inflammation.
Sphingosine-1-Phosphate (S1P) Signaling: S1P is a bioactive lipid mediator that binds to a family of G-protein-coupled receptors (S1PR1-5). It regulates critical processes such as immune cell trafficking, endothelial barrier integrity, and cell proliferation. The S1P concentration gradient between lymphoid tissues (low) and blood/lymph (high), maintained by transporters like SPNS2, guides lymphocyte egress. Targeting S1PR1 with agonists like fingolimod is an established therapy for multiple sclerosis, highlighting the therapeutic relevance of lipid signaling [106].
Eicosanoid Signaling: Phospholipase A2-mediated release of arachidonic acid (AA) from membrane phospholipids initiates the biosynthesis of eicosanoids, including prostaglandins (PGs), thromboxanes (TXs), and leukotrienes (LTs). These signaling molecules have potent and often opposing effects on inflammation, vascular tone, and platelet aggregation. The balance between pro-inflammatory (e.g., PGE2, LTB4) and pro-resolving (e.g., Lipoxins) eicosanoids is critical for controlled immune responses. NSAIDs exert their effects by inhibiting the cyclooxygenase (COX) enzymes in this pathway [106].
Docosahexaenoic Acid (DHA) Signaling: DHA, stored in membrane phospholipids, can be released by calcium-independent phospholipase A2 (iPLA2) and converted to specialized pro-resolving mediators (SPMs) like resolvins and protectins. These SPMs actively orchestrate the resolution of inflammation by reducing neutrophil infiltration, enhancing macrophage phagocytosis of cellular debris, and promoting tissue repair. DHA itself also influences neuronal function by supporting synaptic plasticity, activating antioxidant defenses via NRF2, and inhibiting pro-apoptotic pathways [106].
Therapeutic Implications and Future Directions
The intricate relationship between triglyceride storage and phospholipid-dependent membrane function opens novel therapeutic avenues.
- Lipid Form-Specific Supplementation: Evidence that the phospholipid form of omega-3 PUFAs offers superior bioavailability and metabolic efficacy compared to the triglyceride form suggests a promising nutritional strategy [45] [101]. Phospholipid-based supplements could be more effective in managing hepatic steatosis, adipose tissue inflammation, and insulin resistance.
- Targeting Lipid-Modifying Enzymes: Modulating the activity of enzymes involved in lipid remodeling, such as phospholipases, desaturases, and the enzymes of the TG/FA cycle, presents a potential pharmacological approach. Fine-tuning membrane lipid composition or the rate of lipid cycling could restore metabolic homeostasis.
- Membrane Lipid Therapy (Membrane Lipid Replacement): This emerging concept involves the use of specific phospholipid supplements to restore damaged mitochondrial and cellular membranes, thereby improving cellular function and reducing oxidative stress in aging and disease states.
- Synthetic Biology of Lipids: Advanced techniques could engineer novel lipid molecules or dietary sources (e.g., specific algae strains) that are optimized for both energy partitioning and membrane structuring, offering a highly precise dietary intervention [101].
Future research must focus on translating findings from animal models to human physiology, further elucidating the molecular sensors that link membrane lipid composition to metabolic gene expression, and developing targeted delivery systems for specific lipid entities to relevant tissues.
The biological interplay between triglycerides and phospholipids represents a fundamental metabolic axis. Triglycerides provide essential energy reserves, but their overaccumulation disrupts systemic homeostasis. Phospholipids, by governing membrane structure, fluidity, and signaling capacity, are critical regulators of cellular function and metabolic health. The chemical structure of these dietary lipids directly influences their metabolism, bioavailability, and biological impact. Navigating the metabolic consequences of their imbalance is therefore not merely about limiting fat intake, but about understanding and optimizing the qualitative nature of dietary lipids to maintain the delicate balance between energy storage and membrane health—a balance that is pivotal for preventing and treating modern metabolic diseases.
Comparative Biology and Clinical Validation of Lipid Functions in Health and Disease
In dietary lipid research, the fundamental divergence in the chemical structure of triglycerides and phospholipids dictates their distinct physiological roles, metabolic fates, and ultimately, their impact on human health. While both lipids share a glycerol backbone, their architectural differences orchestrate a clear functional specialization: triglycerides serve as the body's primary energy reservoir, whereas phospholipids are indispensable for constructing cellular membranes and mediating complex signaling cascades [107] [108]. This whitepaper provides an in-depth technical comparison of these two lipid classes, framing the analysis within the context of their chemical structure. We synthesize current research to elucidate how structure dictates function, from bulk energy handling to nanoscale membrane dynamics and cellular communication, providing researchers and drug development professionals with a refined understanding for future innovation.
Structural Foundations: Molecular Blueprint and Physicochemical Properties
The functional chasm between triglycerides and phospholipids is rooted in their distinct molecular architectures.
Core Molecular Structures
- Triglycerides: A triglyceride (or triacylglycerol, TAG) molecule consists of a single glycerol backbone esterified to three fatty acid chains [107] [109]. This structure is overwhelmingly hydrophobic, making triglycerides ideal for forming lipid droplets within adipocytes or for circulating in the bloodstream within the core of lipoprotein particles [108].
- Phospholipids: A phospholipid also features a glycerol backbone. However, it is esterified to two fatty acid chains and one phosphate-containing headgroup [107] [108] [110]. This combination of hydrophobic tails and a hydrophilic head confers an amphipathic nature upon phospholipids, which is the critical property enabling them to spontaneously form the lipid bilayer foundation of all cellular membranes [107] [102].
Impact of Fatty Acid Composition and Molecular Geometry
Beyond the core blueprint, finer structural details impart significant functional nuance.
- Triglyceride Polymorphism: The crystallization and melting behavior of pure triglycerides and complex fat systems are directed by their nanostructure. Slight variations in fatty acid chain length, saturation, and their positioning on the glycerol backbone (stereospecific numbering, sn-position) result in different polymorphic forms (α, β', β). These forms exhibit distinct long and short spacing as revealed by X-ray scattering (XRS), which influences their physical properties, digestibility, and functional performance in food and pharmaceutical systems [8].
- Phospholipid Positional Specificity: The placement of specific fatty acids on the phospholipid's glycerol backbone is critical. For instance, in docosahexaenoic acid-containing phospholipids (PL-DHA), DHA located at the sn-1 position generates a "U-shaped" configuration that significantly affects membrane curvature and fluidity. In contrast, DHA at the sn-2 position maintains a more typical "hairpin" conformation, making it a preferred substrate for phospholipase A₂ (PLA₂) and thereby influencing the production of lipid signaling molecules [26].
Table 1: Fundamental Structural Comparison of Triglycerides and Phospholipids
| Structural Feature | Triglycerides | Phospholipids |
|---|---|---|
| Glycerol Backbone | Present | Present |
| Fatty Acid Chains | 3 | 2 |
| Phosphate Group | Absent | Present (with variable headgroups, e.g., choline, serine) |
| Overall Polarity | Hydrophobic | Amphipathic |
| Primary Molecular Role | Energy Storage | Membrane Matrix & Signaling |
Functional Showdown: Energy Storage vs. Structural and Signaling Roles
The structural differences detailed above manifest in two largely non-overlapping physiological domains.
Triglycerides: Masters of Energy Homeostasis
Triglycerides function as the body's most efficient energy storage system.
- Energy Density and Storage: The highly reduced, carbon-rich nature of their three long-chain fatty acids allows triglycerides to store more than double the energy per gram compared to carbohydrates or proteins [109]. Excess dietary calories are converted into triglycerides and stored in cytoplasmic lipid droplets within white adipocytes [107] [109].
- Mobilization and Use (Lipolysis): During fasting or energy demand, hormones like glucagon and adrenaline activate enzymes such as adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL). These enzymes catalyze the hydrolysis of stored triglycerides, releasing free fatty acids (FFAs) and glycerol into the bloodstream [109]. FFAs are taken up by tissues and undergo mitochondrial β-oxidation to produce ATP, while glycerol can be used for gluconeogenesis in the liver [107] [109].
- Impact of Structure on Digestibility: The molecular structure of a triglyceride directly impacts its metabolic fate. Studies comparing physical mixtures of medium- and long-chain triglycerides (PM) with their enzymatically interesterified counterpart (MLCT) have shown that the MLCT structure, which incorporates both medium- and long-chain fatty acids on the same glycerol molecule, results in a higher degree of lipolysis and greater release of FFAs during in vitro digestion. This structural modification also enhances the bioaccessibility of specific long-chain polyunsaturated fatty acids like EPA and DHA [83].
Phospholipids: Architects of Cellular Integrity and Signaling Hubs
Phospholipids are the cornerstone of cellular structure and key mediators of information flow.
- Forming the Plasma Membrane: Their amphipathic nature drives the spontaneous formation of a stable lipid bilayer in aqueous environments. In this configuration, the hydrophobic tails face inward, shielded from water, while the hydrophilic heads interface with the extracellular fluid and cytosol [107] [108]. This forms a semi-permeable barrier that maintains cellular integrity and regulates the passage of substances.
- Intracellular Signaling and Second Messengers: Phospholipids are dynamic signaling molecules. Upon activation of cell surface receptors, enzymes like phospholipase C (PLC) hydrolyze phosphatidylinositol 4,5-bisphosphate (PIP₂) to generate the second messengers inositol trisphosphate (IP₃) and diacylglycerol (DAG), which mediate calcium release and protein kinase C activation, respectively [102].
- Influence on Protein Aggregation and Disease: The lipid composition of cellular membranes can dramatically alter the pathological aggregation of proteins. For example, α-synuclein fibrils formed in the presence of the phospholipid cardiolipin (CL) induce a significantly stronger unfolded protein response (UPR) in the endoplasmic reticulum of rat dopaminergic cells compared to fibrils formed in a lipid-free environment or with phosphatidylcholine (PC). This suggests that specific phospholipids can directly modify the toxicity of protein aggregates linked to neurodegenerative diseases like Parkinson's [111].
Table 2: Head-to-Head Functional Comparison in a Biological Context
| Functional Aspect | Triglycerides | Phospholipids |
|---|---|---|
| Primary Biological Role | Long-term energy storage | Cell membrane structure; Signaling |
| Localization in Body | Adipose tissue; Lipid droplets; Bloodstream (in lipoproteins) | All cellular membranes; Organelles |
| Metabolic Pathway | Lipogenesis; Lipolysis | De novo synthesis; Phospholipase-mediated remodeling; Signal transduction |
| Key Regulatory Enzymes | Hormone-sensitive lipase (HSL), ATGL | Phospholipase A₂, C, D (PLA₂, PLC, PLD) |
| Link to Human Disease | Obesity, Insulin resistance, Cardiovascular disease | Neurodegenerative disorders, Cancer cell signaling |
Experimental Insights: Methodologies for Functional Analysis
Investigating Triglyceride Digestibility and Bioaccessibility
Objective: To evaluate how the triglyceride structure (e.g., PM vs. MLCT) affects its digestion kinetics and the bioaccessibility of its fatty acid components [83].
Protocol:
- Sample Preparation: Prepare the test lipids (e.g., PM and MLCT) with identical total fatty acid compositions.
- In Vitro Digestion Simulation: Subject the lipids to a standardized in vitro digestion model (e.g., INFOGEST protocol) that simulates gastric and intestinal phases.
- Lipolysis Monitoring: Continuously titrate the reaction mixture to maintain pH, recording the volume of base consumed to neutralize FFAs released by pancreatic lipase action.
- Analysis:
- Lipolysis Degree: Calculate the percentage of total fatty acids released as FFAs.
- Kinetic Rate Constant: Fit the FFA release data to a first-order kinetic model to determine the apparent rate constant.
- Bioaccessibility: After digestion, isolate the micellar phase via ultracentrifugation. Analyze its lipid composition using gas chromatography (GC) to determine the bioaccessibility of specific fatty acids like DHA and EPA [83].
Profiling Lipid-Induced Toxicity in Protein Aggregation
Objective: To determine how phospholipids alter the secondary structure and cell toxicity of amyloid fibrils (e.g., α-synuclein) [111].
Protocol:
- Fibril Formation: Incubate the amyloidogenic protein (α-synuclein) under aggregating conditions in the presence of different phospholipids (e.g., PC, CL, PC:Cho mixtures) and a lipid-free control.
- Structural Characterization:
- Atomic Force Microscopy Infrared (AFM-IR): Use a metalized AFM tip to obtain IR spectra from individual fibrils. Deconvolute the amide I region to quantify the percentage of parallel β-sheet, antiparallel β-sheet, and unordered structure.
- Fourier-Transform Infrared (FTIR) and Circular Dichroism (CD): Perform bulk analysis for secondary structure confirmation.
- Cell Toxicity Assay:
- Expose rat dopaminergic neuronal cells (e.g., N27 cells) to the pre-formed fibrils.
- Use real-time quantitative PCR (rt-qPCR) to measure the expression levels of key markers of the unfolded protein response (UPR) in the endoplasmic reticulum (e.g., PERK, ATF4, CHOP) and mitochondria over time (e.g., 6, 12, 24 hours) [111].
Pathway Visualization and Logical Workflows
Phospholipid-Dependent UPR Activation Pathway
The following diagram visualizes the key signaling pathway through which α-synuclein fibrils, formed in the presence of specific phospholipids, trigger cellular stress responses.
Diagram Title: Phospholipid-Modified α-Syn Fibrils Activate ER Stress
Experimental Workflow for Triglyceride Digestibility Analysis
This flowchart outlines the key steps in the methodology for analyzing the digestibility of different triglyceride structures.
Diagram Title: Triglyceride Digestibility Assay Workflow
The Scientist's Toolkit: Essential Research Reagents and Solutions
Table 3: Key Reagents and Materials for Lipid Functional Analysis
| Research Reagent / Material | Function / Application | Specific Example from Literature |
|---|---|---|
| Porcine Pancreatic Lipase | Key enzyme for in vitro simulation of intestinal lipid digestion, hydrolyzing triglycerides at the sn-1 and sn-3 positions. | Used to compare lipolysis of PM vs. MLCT [83]. |
| Structured Lipids (e.g., MLCT) | Model compounds to study the effect of triglyceride molecular structure (fatty acid positioning) on digestion, absorption, and metabolism. | Synthesized via enzymatic interesterification for digestibility studies [83]. |
| Defined Phospholipids (PC, CL, PS) | Used to investigate how specific membrane lipid environments influence protein-lipid interactions, protein aggregation, and cell signaling pathways. | Incubated with α-synuclein to form structurally and toxicologically distinct fibrils [111]. |
| rt-qPCR Assays for UPR Markers | Quantitative measurement of gene expression changes in response to cellular stress (e.g., ER stress induced by toxic protein aggregates). | Used to profile PERK, ATF4, and CHOP expression in N27 cells [111]. |
| AFM-IR (Atomic Force Microscopy - Infrared Spectroscopy) | Nanoscale structural analysis technique that combines topography mapping with chemical identification; ideal for characterizing heterogeneous samples like protein-lipid aggregates. | Used to determine the secondary structure of individual α-synuclein fibrils formed with different lipids [111]. |
| X-ray Scattering (SAXS/WAXS) | Analysis of nanostructural aspects, polymorphic states, and phase transitions in pure and complex triglyceride crystal systems. | Used to determine lamellar stacking and hydrocarbon chain packing in fats [8]. |
The head-to-head comparison unequivocally demonstrates that the chemical structure of dietary triglycerides and phospholipids is the principal determinant of their biological fate. Triglycerides, with their simple, hydrophobic triad of fatty acids, are optimized for dense energy storage and on-demand mobilization. In contrast, the amphipathic design of phospholipids predestines them for roles that triglycerides cannot fulfill: forming the architectural fabric of cells and serving as dynamic platforms and precursors for intricate signaling networks. For researchers and drug developers, this structural-functional dichotomy is paramount. Tailoring triglyceride structures can optimize energy delivery and metabolic health, while manipulating phospholipid composition offers a pathway to influence membrane-associated processes in neurodegeneration, cancer, and beyond. The future of dietary lipid research lies in a deeper, nano-scale understanding of these structures to design novel lipids for targeted nutritional and therapeutic interventions.
The absorption route of dietary lipids and lipophilic compounds fundamentally dictates their subsequent metabolic fate, systemic distribution, and biological activity. While hydrophilic molecules typically enter the portal vein and undergo first-pass hepatic metabolism, highly lipophilic substances preferentially access the systemic circulation via the intestinal lymphatic system, bypassing initial liver exposure. This divergence is primarily governed by the physicochemical properties of the molecule, notably its lipophilicity and lipid solubility, as well as the chemical structure of co-administered lipids, including triglyceride configuration and phospholipid composition. Understanding these pathways is critical for optimizing drug delivery systems and assessing the tissue distribution and toxicological profiles of both pharmaceuticals and environmental toxins. This review synthesizes current knowledge on the mechanisms governing portal versus lymphatic transport, with a specific focus on the context of dietary lipid research, and provides detailed experimental methodologies for their investigation.
The gastrointestinal tract presents two principal routes for the absorption of substances into the systemic circulation: the portal vein and the intestinal lymphatic system [112]. The majority of absorbed materials, including most nutrients and hydrophilic drugs, enter the portal blood due to its high flow rate, which is approximately 500-fold greater than that of intestinal lymph [112]. Subsequently, these compounds are directed to the liver, where they may undergo significant first-pass metabolism before reaching the systemic circulation.
In contrast, the intestinal lymphatics offer an alternative pathway that is particularly crucial for the absorption of highly lipophilic compounds. This route provides direct access to the systemic circulation via the thoracic duct, thereby circumventing first-pass hepatic metabolism [112]. The physiological basis for this selective transport lies in the architecture of the capillary beds. Blood capillaries possess a continuous, relatively impermeable endothelium, whereas lymphatic capillaries (lacteals) are more permeable, allowing the entry of larger colloidal structures such as chylomicrons and other lipoproteins [112] [113]. The preferential access of lipoproteins to the lymphatics is dictated by their physical size, which limits diffusion across the vascular endothelium [112].
Determinants of Absorption Route: Lipophilicity and Lipid Solubility
The primary factor determining the route of absorption for a compound is its lipophilicity, commonly quantified by its octanol/water partition coefficient (Log P). A strong correlation exists between increasing Log P and the propensity for lymphatic transport.
Table 1: Impact of Lipophilicity on Absorption Route of Select Compounds
| Compound | Log P | Primary Absorption Route | Key Findings |
|---|---|---|---|
| Caffeine | Low (Hydrophilic) | Portal Vein | Only very small quantities recovered in lymph [112]. |
| Pentachlorophenol (PCP) | 3.5 | Portal Vein (Predominant) | Lower lipophilicity favors portal absorption [114]. |
| Hexachlorobenzene | 5.7 | Mixed (Lymphatic & Portal) | Intermediate lipophilicity leads to dual pathway absorption [114]. |
| DDT | 6.91 | Lymphatic (Predominant) | High lipophilicity promotes association with chylomicrons and lymphatic transport [112] [114]. |
| DDE | 6.96 | Lymphatic (Predominant) | Similar to DDT, high Log P drives lymphatic retrieval [114]. |
General thresholds have been established for lymphatic transport: typically, compounds require a Log P > 5 and long-chain triglyceride solubility > 50 mg/g [112]. This is because lymphatic transport is not primarily driven by the compound's passive permeability, but by its ability to associate with intracellular lipoproteins within the enterocyte [112]. Post-absorption, highly lipophilic molecules dissolve in the apolar lipid core of assembling chylomicrons [112]. The subsequent size of these lipoproteins (ranging from 75 to 600 nm) precludes their entry into the tight blood capillaries, forcing their exclusive entry into the more permeable lacteals [112].
The Critical Role of Dietary Lipid Structure
The efficiency of lymphatic drug transport is profoundly influenced by the source, type, and structure of co-administered dietary lipids, as these factors drive the formation and composition of chylomicrons.
Fatty Acid Chain Length and Saturation
- Chain Length: Long-chain fatty acids (≥ C14) and their triglycerides are pivotal for efficient lymphatic transport. They are extensively incorporated into resynthesized triglycerides and packaged into chylomicrons, with 40-60% of the lipid dose transported via the lymph [112]. In contrast, medium-chain and short-chain fatty acids are more water-soluble and are primarily absorbed directly into the portal blood [112] [115].
- Degree of Saturation: Unsaturated fatty acids (MUFAs and PUFAs) generally promote lymphatic lipid and drug transport more effectively than their saturated counterparts. They produce larger lipoproteins and enhance the lymphatic transport of associated drugs [112].
Positional Distribution on the Glycerol Backbone
The stereospecific position of fatty acids on the dietary triglyceride molecule significantly influences absorption and subsequent chylomicron composition. During digestion, fatty acids in the sn-1 and sn-3 positions are typically hydrolyzed, releasing free fatty acids, while the fatty acid in the sn-2 position is retained as a 2-monoglyceride [115]. These 2-monoglycerides are preferentially used in the resynthesis of new triglycerides within the enterocyte. Consequently, the absorption of fatty acids originally located at the sn-2 position is favored, and they are directly incorporated into the lymphatic system via chylomicrons [115] [116]. This positional distribution has been shown to affect fasting blood lipoprotein concentrations in humans, though the effects may be modest [116].
Phospholipids as Bioenhancers
Phospholipids, particularly phosphatidylcholine (PC) and its digestion product lysophosphatidylcholine (LPC), play a significant role in enhancing lymphatic transport. They act as endogenous emulsifiers and facilitate the formation of lipoproteins. LPC has been demonstrated to enhance the lymphatic transport of compounds like halofantrine and α-tocopherol [112]. Their amphipathic nature allows them to integrate into lipid membranes and mixed micelles, improving the solubilization and absorption of lipophilic drugs [4].
Advantages and Consequences of Lymphatic Transport
The choice of absorption pathway has profound implications for the pharmacokinetics and pharmacodynamics of a substance.
- Bypass of First-Pass Metabolism: Transport via the intestinal lymphatics allows drugs to enter the systemic circulation directly without first passing through the liver. This can lead to a significant enhancement in oral bioavailability for compounds susceptible to high hepatic extraction [112] [117].
- Targeted Drug Delivery: The lymphatic system is the primary route for the metastasis of many solid tumors and is critically involved in HIV infection and other immune processes. Lymphatic delivery of anticancer or antiviral drugs can, therefore, result in higher drug concentrations at the disease site [112].
- Altered Toxicity Profiles: Changes in the distribution pathway can lead to altered tissue exposure, potentially modifying a drug's toxicological profile. Lymphatic transport results in a distinct pattern of local drug exposure to the lymphatics and a changed mode of delivery to the systemic circulation [112].
Experimental Protocols for Investigating Absorption Routes
Quantifying the contribution of each absorption pathway requires sophisticated surgical models. The following describes a definitive in vivo protocol for the simultaneous measurement of portal and lymphatic absorption.
Surgical Cannulation Model in Rats
This method allows for the direct and quantitative comparison of drug appearance in both the lymph and the portal blood [114].
Materials and Reagents:
- Animals: Adult male Sprague-Dawley rats (∼350 g).
- Test Compounds: Radiolabeled compounds (e.g., [¹⁴C]DDT, [¹⁴C]Hexachlorobenzene).
- Portal Absorption Marker: [³H]3-O-methyl glucose (3-OMG), a non-metabolized sugar absorbed via the portal vein.
- Lipid Vehicle: Long-chain triglyceride oil (e.g., olive oil).
- Cannulas: Micro-Renathane (e.g., MRE-033, OD 0.033 inches) for portal vein cannulation.
- Anesthetics & Analgesics: Isoflurane for anesthesia; Buprenex for postoperative analgesia.
Procedure:
Portal Vein Cannulation: Under isoflurane anesthesia, a ventral midline incision is made to expose the abdominal viscera. The ileocolic vein is identified, and a cannula is inserted and advanced into the portal vein. The cannula is heparinized, sealed, and placed inside the peritoneal cavity. The incision is closed in layers, and the animal is allowed to recover for 2-3 days with analgesic support [114].
Mesenteric Lymphatic Duct Cannulation: After recovery, the animal is fasted overnight and anesthetized again. The major mesenteric lymph duct is cannulated according to the method of Bollman, Cain, and Grindlay. A soft silicone duodenal infusion tube is also inserted via the fundus of the stomach and secured in the duodenum [114].
Post-operative Care and Dosing: The animal is placed in a restraining cage (Bollman cage) and infused with a saline-glucose solution to maintain hydration and lymph flow. After an overnight recovery, the test compound dissolved in oil and the 3-OMG marker in an aqueous solution are administered as bolus doses via the duodenal tube [114].
Sample Collection:
- Lymph: Collected continuously for a set period (e.g., 6 hours) into chilled tubes.
- Portal Blood: Serial samples (e.g., 100 μL) are drawn from the portal vein cannula at multiple time points.
- Peripheral Blood: May also be collected from a separate peripheral vessel for comparison.
Data Analysis:
- Lymphatic Transport: Quantified by the cumulative percentage of the administered dose recovered in the lymph over time.
- Portal Absorption: Calculated by comparing the Area Under the Curve (AUC) of the test compound in portal blood to the AUC of the portal absorption marker, 3-OMG. Since lymph is diverted, appearance in portal blood is solely due to portal absorption [114].
The following workflow diagram illustrates this experimental protocol:
Figure 1: Experimental Workflow for Simultaneous Portal and Lymphatic Measurement
The Scientist's Toolkit: Key Research Reagents
Table 2: Essential Reagents for Absorption and Lymphatic Transport Studies
| Reagent / Material | Function & Rationale |
|---|---|
| Radiolabeled Compounds (e.g., ¹⁴C, ³H) | Enable precise, quantitative tracking of the compound of interest through different absorption pathways and its distribution in tissues. |
| 3-O-Methyl Glucose ([³H]OMG) | Serves as a non-metabolizable portal absorption marker. Its appearance in portal blood provides a benchmark for quantifying portal absorption of co-administered test compounds [114]. |
| Long-Chain Triglyceride Oil (e.g., Olive Oil) | A lipid vehicle that stimulates chylomicron formation, thereby promoting the lymphatic pathway for highly lipophilic compounds [112] [114]. |
| Micro-Renathane Cannulas | Biocompatible tubing used for cannulating delicate vessels like the portal vein and mesenteric lymph duct, ensuring patency during extended experiments [114]. |
| Bollman Restraining Cages | Specialized cages that allow limited movement while protecting exteriorized cannulas, enabling continuous lymph collection from conscious, unrestrained animals [114]. |
Implications for Drug Delivery and Toxicity
The principles of lymphatic transport are actively leveraged in pharmaceutical sciences, particularly for the delivery of challenging drug molecules.
- Lipid-Based Formulations: Self-emulsifying drug delivery systems (SEDDS) and lipid solutions are designed to present the drug in a pre-solubilized state within a lipid matrix, thereby promoting drug association with intestinal lipoproteins and enhancing lymphatic transport [117]. The choice of lipid (long-chain vs. medium-chain) and emulsifiers (e.g., phospholipids) is critical for success.
- Lipidic Prodrugs: Covalently coupling a drug to a lipid moiety (e.g., a fatty acid, diglyceride, or phosphoglyceride) is a strategy to enhance lipophilicity and promote incorporation into the lipid resynthetic pathways within the enterocyte. Glyceride-based prodrugs mimic dietary lipids and often show superior lymphatic targeting compared to simple esters [112].
- Toxicokinetics of Environmental Toxins: The absorption pathway significantly influences the body's handling of lipophilic environmental contaminants. For instance, the lymphatic absorption of organochlorine compounds like DDT and hexachlorobenzene delivers these toxins directly to systemic tissues, bypassing potential hepatic detoxification and leading to sequestration in adipose tissue [114]. This can result in chronic exposure and bioaccumulation.
The following diagram summarizes how chemical structure dictates the metabolic fate of a compound through these divergent pathways:
Figure 2: Decision Flow of Absorption Pathways
The divergence between portal and lymphatic transport is a fundamental physiological process with extensive implications for the bioavailability, tissue distribution, and efficacy/toxicity of lipophilic molecules. The chemical structure of dietary lipids—including fatty acid chain length, degree of saturation, and positional distribution on the triglyceride backbone—serves as a critical switch directing compounds towards one pathway or the other. A deep understanding of these mechanisms, coupled with robust experimental models for their study, provides a powerful framework for advancing strategies in drug delivery, as well as for assessing the health risks posed by lipophilic environmental chemicals. Future research will continue to refine our understanding of intracellular lipid trafficking and lipoprotein assembly, offering new opportunities for precise targeting of the intestinal lymphatic system.
Cardiometabolic diseases represent a significant global health burden, with dyslipidemia serving as a cornerstone of pathogenesis. This in-depth technical review examines the dichotomous roles of two critical lipid classes: triglycerides (TG) as established risk factors and phospholipids as fundamental protective components in lipoprotein metabolism and hepatic function. Framed within the context of chemical structure-function relationships, we explore how the distinct molecular architectures of dietary triglycerides and phospholipids dictate their metabolic fates and biological activities. Through comprehensive analysis of current literature and multi-omics data, this review provides researchers and drug development professionals with structured quantitative data, experimental methodologies, and molecular pathway visualizations to advance therapeutic innovation in cardiometabolic disorders.
The biological behavior of lipids is fundamentally governed by their chemical structure. Triglycerides, consisting of three fatty acids esterified to a glycerol backbone, are highly hydrophobic molecules that serve as compact energy stores [28] [41]. Their complete insolubility in water necessitates packaging into lipoprotein particles for transport through the aqueous plasma environment [52]. In contrast, phospholipids such as phosphatidylcholine (PC) and phosphatidylethanolamine (PE) feature a glycerol backbone with two fatty acid chains and a phosphate-containing polar head group [118] [41]. This amphipathic nature enables phospholipids to form membrane bilayers and act as emulsifiers, facilitating the solubilization and transport of hydrophobic lipids [28].
The structural dichotomy between these lipid classes establishes their divergent roles in cardiometabolic health: triglycerides as risk biomarkers and energy substrates versus phospholipids as structural components and metabolic regulators. Understanding this structure-function relationship is crucial for developing targeted interventions for cardiometabolic diseases.
Triglycerides as Cardiovascular Risk Factors: Evidence and Mechanisms
Epidemiological Evidence and Population-Specific Risk Profiles
Recent large-scale cohort studies have revealed important population-specific associations between triglyceride levels and mortality risk. The ChinaHEART study, encompassing 3.8 million Chinese adults, demonstrated distinctive patterns compared to Western populations [119].
Table 1: Triglyceride-Associated Mortality Hazard Ratios by Population
| Population Group | TG Quintile | All-Cause Mortality HR (95% CI) | CVD Mortality HR (95% CI) |
|---|---|---|---|
| Chinese Men | Q2 | 1.07 (1.05-1.10) | 1.12 (1.08-1.16) |
| Q3 | 1.12 (1.09-1.15) | 1.20 (1.16-1.25) | |
| Q4 | 1.14 (1.11-1.17) | 1.25 (1.20-1.30) | |
| Q5 | 1.15 (1.12-1.18) | 1.28 (1.23-1.33) | |
| Chinese Women | Q2 | 0.94 (0.91-0.97) | 0.95 (0.90-1.00) |
| Q3 | 0.95 (0.92-0.98) | 0.97 (0.92-1.02) | |
| Q4 | 1.00 (0.97-1.03) | 1.05 (1.00-1.11) | |
| Q5 | 1.09 (1.05-1.12) | 1.18 (1.12-1.24) | |
| White Men | Q2 | - | 1.02 (0.97-1.07) |
| Q3 | - | 1.05 (1.00-1.11) | |
| Q4 | - | 1.07 (1.02-1.22) | |
| Q5 | - | 1.19 (1.13-1.24) | |
| White Women | Q2 | - | 1.14 (1.08-1.21) |
| Q3 | - | 1.17 (1.11-1.24) | |
| Q4 | - | 1.26 (1.18-1.33) | |
| Q5 | - | 1.41 (1.33-1.50) |
Notably, the study revealed that all-cause and cardiovascular disease (CVD) mortality in Chinese men increased abruptly with even modest TG elevation then plateaued, while Chinese women exhibited U-shaped associations [119]. This contrasts with White populations, where CVD risk increases incrementally with TG levels. These findings suggest that conventionally low-to-moderate TG ranges may confer significant risk in Chinese and other East Asian populations, implicating potential needs for population-specific intervention strategies.
Molecular Mechanisms of Triglyceride-Rich Lipoprotein Metabolism
The atherogenicity of triglycerides is primarily mediated through their transport in triglyceride-rich lipoproteins (TRLs), including chylomicrons and very-low-density lipoproteins (VLDL) [103]. The metabolism of these particles involves a complex series of assembly, secretion, and processing steps.
Table 2: Characteristics of Major Lipoprotein Classes
| Lipoprotein | Density (g/ml) | Size (nm) | Primary Lipids | Major Apoproteins | Primary Origin |
|---|---|---|---|---|---|
| Chylomicrons | <0.930 | 75-1200 | Dietary Triglycerides | Apo B-48, Apo C, Apo E | Intestine |
| VLDL | 0.930-1.006 | 30-80 | Endogenous Triglycerides | Apo B-100, Apo E, Apo C | Liver |
| IDL | 1.006-1.019 | 25-35 | Triglycerides, Cholesterol | Apo B-100, Apo E | VLDL catabolism |
| LDL | 1.019-1.063 | 18-25 | Cholesterol | Apo B-100 | IDL catabolism |
| HDL | 1.063-1.210 | 5-12 | Cholesterol, Phospholipids | Apo A-I, Apo A-II | Liver, Intestine |
Hepatic VLDL Assembly and Secretion
VLDL assembly occurs through a sophisticated lipidation process of apoB100 in the liver [103]. The process begins with cotranslational transfer of triglycerides to nascent apoB polypeptides in the rough endoplasmic reticulum, mediated by microsomal triglyceride-transfer protein (MTP). This initial lipidation stabilizes the nascent apoB polypeptide, forming a pre-VLDL particle [103]. The immature pre-VLDL subsequently undergoes further lipidation in the secretory pathway, forming triglyceride-poor VLDL2 particles. These can either be secreted directly or undergo additional lipidation through fusion with cytoplasmic lipid droplets to form large, triglyceride-rich VLDL1 particles [103]. This bulk addition of triglycerides is highly regulated, with studies showing that subjects with type 2 diabetes secrete more—not larger—VLDL1 particles than non-diabetic controls [103].
Intestinal Chylomicron Assembly
Chylomicron assembly in enterocytes parallels VLDL formation but utilizes apoB48, which corresponds to the N-terminal 48% of apoB100 generated through mRNA editing [52] [103]. This process requires MTTP and Sar1 GTPase, critical for intracellular transport of apoB48-containing particles from the ER to the Golgi [103]. Newly synthesized chylomicrons carrying dietary lipids are secreted through lacteal endothelial gaps into the lymphatic system, bypassing the hepatic portal system [103].
Lipoprotein Processing and Remnant Formation
Following secretion, triglycerides in both chylomicrons and VLDL are hydrolyzed by lipoprotein lipase (LPL) in peripheral tissues, releasing free fatty acids for storage or energy production [28] [52]. This process generates cholesterol-enriched remnant particles (chylomicron remnants and IDL), which are highly atherogenic [52] [103]. Remnant particles are typically cleared by hepatic receptors, but prolonged circulation promotes arterial infiltration and atherosclerosis development.
Figure 1: Metabolic Pathway of Triglyceride-Rich Lipoproteins and Atherogenesis
Phospholipids in Hepatic Protection and Metabolic Regulation
Phosphatidylcholine and Liver Homeostasis
Phosphatidylcholine (PC), the predominant phospholipid in mammalian membranes, plays critical roles in hepatic lipid metabolism and protection against steatosis. The PC/PE ratio in cellular membranes serves as a key regulator of membrane integrity and metabolic function [118]. Abnormally low hepatic PC/PE ratios impair membrane stability and are associated with non-alcoholic fatty liver disease (NAFLD), now redefined as metabolic dysfunction-associated steatotic liver disease (MASLD) [120] [118].
Essential phospholipids (EPLs), particularly those rich in phosphatidylcholine (72-96%), demonstrate significant efficacy in reducing liver steatosis [120]. The quantitatively dominant molecule in soybean-derived EPLs is 1,2-dilinoleoylphosphatidylcholine (DLPC), representing up to 52% of administered phosphatidylcholine molecules [120]. This high DLPC content distinguishes EPLs from typical unprocessed phospholipids and accounts for their enhanced biological activity.
Molecular Mechanisms of Phospholipid-Mediated Hepatoprotection
Phospholipids exert hepatoprotective effects through multiple molecular pathways:
1. VLDL Assembly and Secretion: PC is essential for VLDL assembly, as it comprises the surface monolayer of nascent particles. Inhibition of hepatic PC synthesis impairs VLDL secretion, leading to intracellular triglyceride accumulation [118]. PC availability regulates the microsomal triglyceride transfer protein (MTP) activity, crucial for lipoprotein assembly [118].
2. Membrane Integrity and Signaling: The PC/PE ratio governs membrane fluidity, permeability, and the activity of membrane-bound enzymes and receptors [118]. Abnormal PC/PE ratios disrupt endoplasmic reticulum homeostasis and promote unfolded protein response activation, contributing to insulin resistance and metabolic dysfunction [118].
3. Reverse Cholesterol Transport: Phospholipids in HDL particles facilitate cholesterol efflux from peripheral tissues, including macrophage foam cells in atherosclerotic lesions [52]. This process, known as reverse cholesterol transport, represents a key anti-atherogenic mechanism [52].
4. Mitochondrial Function: The mitochondrial membrane phospholipid cardiolipin plays critical roles in oxidative phosphorylation and energy production [121]. Alterations in cardiolipin metabolism impair mitochondrial function and promote hepatic steatosis [121].
Figure 2: Phospholipid-Mediated Hepatoprotective Mechanisms
Experimental Approaches and Methodologies
Metabolomic Profiling in Preclinical Atherosclerosis
Comprehensive metabolomic analyses provide powerful insights into lipid metabolism alterations in cardiometabolic diseases. A recent study investigating preclinical atherosclerosis (PCA) employed rigorous methodologies for metabolic profiling [122].
Table 3: Key Biochemical Parameters in Preclinical Atherosclerosis
| Parameter | Control Group | PCA Group | p-value | Assay Method |
|---|---|---|---|---|
| Triglycerides (mmol/L) | 1.25 ± 0.31 | 1.89 ± 0.42 | <0.001 | Enzymatic colorimetric |
| LDL-C (mmol/L) | 2.45 ± 0.56 | 3.12 ± 0.61 | <0.001 | Homogeneous assay |
| HDL-C (mmol/L) | 1.42 ± 0.35 | 1.21 ± 0.29 | <0.01 | Homogeneous assay |
| Fasting Glucose (mmol/L) | 5.12 ± 0.45 | 5.89 ± 0.62 | <0.001 | Hexokinase method |
| Uric Acid (μmol/L) | 285.3 ± 52.1 | 335.7 ± 61.8 | <0.01 | Uricase-POD method |
| ApoA1 (g/L) | 1.38 ± 0.21 | 1.25 ± 0.19 | <0.05 | Immunoturbidimetry |
| ApoB (g/L) | 0.82 ± 0.16 | 0.98 ± 0.18 | <0.001 | Immunoturbidimetry |
LC-MS/MS Metabolomic Protocol
Sample Preparation:
- Fasting venous blood collected in K3EDTA vacuum tubes
- Centrifugation at 4°C, 3000 rpm for 10 minutes
- Plasma aliquoting (100μL) and protein precipitation with pre-chilled 80% methanol
- Incubation on ice (5 min), centrifugation at 15,000 × g, 4°C for 20 minutes
- Supernatant dilution to 53% methanol with LC-MS grade water
- Secondary centrifugation at 15,000 × g, 4°C for 20 minutes [122]
UHPLC-MS/MS Analysis:
- System: Vanquish UHPLC coupled with Orbitrap Q Exactive HF mass spectrometer
- Column: Hypersil Gold (100 × 2.1 mm, 1.9 μm)
- Mobile Phase: Eluent A (0.1% formic acid in water), Eluent B (methanol)
- Gradient: 12-min linear gradient at 0.2 mL/min
- MS Parameters: Spray voltage 3.5 kV, capillary temperature 320°C, sheath gas 35 psi
- Mass Range: m/z 100-1000 for both MS1 and MS2 [122]
Data Processing:
- Raw data processed using Compound Discoverer 3.3
- Peak alignment, picking, and quantitation with mass tolerance of 5 ppm
- Metabolite annotation via mzCloud, mzVault, and MassList databases
- Statistical analysis using R (v3.4.3), Python (v2.7.6), and CentOS [122]
This approach identified 105 differential metabolites in positive ion mode (29 upregulated, 76 downregulated) and 105 in negative ion mode (39 upregulated, 66 downregulated) in PCA patients, primarily involving lipid metabolism, inflammation-mediated processes, and amino acid metabolism [122].
Multi-Omics Integration in MASLD Research
Advanced multi-omics approaches enable comprehensive understanding of phospholipid metabolism in hepatic disorders. A recent study investigating metabolic dysfunction-associated steatotic liver disease (MASLD) integrated lipidomics and transcriptomics methodologies [121].
Experimental Model:
- C57BL/6J mice fed high-fat diet (HFD) for 12 weeks
- Control group fed normal diet
- Biochemical analysis of serum and liver tissues
- H&E staining for histological assessment [121]
Lipidomic Analysis:
- Comprehensive profiling of 49 lipid classes and 3221 lipid species
- Identification of significant alterations in PC, TG, PE, and cardiolipin metabolism
- Detection of differentially expressed genes (678 total: 364 upregulated, 314 downregulated) [121]
Key Findings:
- KEGG enrichment analysis revealed downregulation of Gpat4, Gpcpd1, Chkb, and Etnppl genes
- These genes are involved in glycerophospholipid metabolism and PC biosynthesis
- Disruption of PC/PE ratio associated with mitochondrial dysfunction and impaired VLDL secretion [121]
Figure 3: Integrated Multi-Omics Experimental Workflow
The Scientist's Toolkit: Essential Research Reagents and Methodologies
Table 4: Essential Research Reagents and Materials for Lipid Metabolism Studies
| Reagent/Material | Specifications | Primary Research Application | Key Suppliers |
|---|---|---|---|
| UHPLC-MS/MS System | Vanquish UHPLC with Orbitrap Q Exactive HF mass spectrometer | Metabolite identification and quantification | Thermo Fisher Scientific |
| Phospholipid Standards | Essential phospholipids (72-96% phosphatidylcholine), 1,2-dilinoleoylphosphatidylcholine (DLPC) | Phospholipid metabolism studies, membrane biology | Sigma-Aldrich, Avanti Polar Lipids |
| Lipoprotein Lipase | Recombinant human LPL, activity >50,000 U/mg | TRL metabolism studies, lipolysis assays | R&D Systems, Merck Millipore |
| Apolipoprotein Assays | Anti-apoB100, Anti-apoB48, Anti-apoA-I antibodies | Lipoprotein characterization, ELISA development | Abcam, Thermo Fisher |
| MTP Inhibitors | Small molecule inhibitors (e.g., Lomitapide), IC50 <10 nM | VLDL assembly studies, lipid transfer assays | Cayman Chemical, MedChemExpress |
| Phospholipid Biosynthesis Assays | Radiolabeled choline (³H-choline), ethanolamine precursors | PC/PE metabolism tracking, kinetic studies | American Radiolabeled Chemicals |
| Lipoprotein Density Kits | Sequential ultracentrifugation kits, density range 0.92-1.21 g/mL | Lipoprotein fraction isolation and characterization | Beckman Coulter, Sigma-Aldrich |
The structural dichotomy between triglycerides and phospholipids underpins their contrasting roles in cardiometabolic health. Triglycerides, as hydrophobic energy storage molecules, contribute to disease risk through their transport in atherogenic lipoprotein particles and the generation of cholesterol-enriched remnants. Phospholipids, particularly phosphatidylcholine, serve essential structural and regulatory functions that protect against hepatic steatosis and promote reverse cholesterol transport.
Future research directions should focus on:
- Population-Specific Therapeutics: Developing tailored interventions based on ethnic variations in triglyceride-associated risk [119]
- Phospholipid-Based Therapeutics: Optimizing essential phospholipid formulations for MASLD treatment [120]
- Multi-Omics Integration: Advanced computational approaches to integrate lipidomic, transcriptomic, and proteomic data for personalized risk prediction [121]
- Membrane Lipid Therapy: Targeted modulation of PC/PE ratios as a novel approach for metabolic disorders [118]
Understanding the chemical structure-function relationships of dietary triglycerides and phospholipids provides a robust foundation for developing next-generation therapeutics aimed at combating the global burden of cardiometabolic diseases.
Phospholipids are indispensable structural and functional components of neuronal membranes, serving as critical determinants of brain integrity, synaptic plasticity, and cognitive function. This whitepaper synthesizes current research elucidating the mechanistic roles of specific phospholipid classes in regulating membrane fluidity, receptor dynamics, and neurotransmission pathways. Evidence from lipidomic studies, genetic models, and dietary interventions demonstrates that phospholipid composition directly influences neurological health, with alterations linked to cognitive decline, mood disorders, and neurodegenerative conditions. The complex interplay between dietary phospholipid sources, molecular structure, and neuronal function offers promising avenues for therapeutic development and precision nutrition strategies targeting brain disorders.
The human brain is the second most lipid-rich organ in the body, with phospholipids constituting approximately 60% of its dry weight. These amphipathic molecules form the fundamental architectural matrix of all neuronal membranes, creating specialized microdomains that facilitate compartmentalization, signal transduction, and synaptic communication. The brain's three predominant phospholipids—phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylserine (PS)—account for approximately 35-40%, 35-40%, and 20% of total brain phospholipids, respectively [123]. Mitochondrial membranes exhibit even greater phospholipid diversity, containing not only PC, PE, and PS but also phosphatidylinositol (PI), phosphatidic acid (PA), and the mitochondria-specific cardiolipin (CL) and phosphatidylglycerol (PG) [123].
The chemical structure of phospholipids confers unique biophysical properties essential for neuronal function. Each phospholipid consists of a hydrophilic head group containing a phosphate moiety and a hydrophobic tail typically composed of two fatty acid chains. The fatty acid composition, particularly the degree of unsaturation, critically determines membrane fluidity, with polyunsaturated fatty acids (PUFAs) introducing kinks that increase membrane flexibility and permeability [123]. The most common PUFAs in neuronal phospholipids include docosahexaenoic acid (DHA, 22:6n-3) and arachidonic acid (AA, 20:4n-6), which serve as precursors for bioactive lipid mediators involved in neuroinflammation and resolution pathways [123] [124].
Table 1: Major Phospholipid Classes in Neuronal Membranes
| Phospholipid Class | Abbreviation | Approximate Brain Percentage | Primary Neuronal Functions |
|---|---|---|---|
| Phosphatidylcholine | PC | 35-40% | Major structural component, precursor for acetylcholine and signaling molecules |
| Phosphatidylethanolamine | PE | 35-40% | Membrane fusion, autophagy, mitochondrial function |
| Phosphatidylserine | PS | ~20% | Apoptotic signaling, membrane trafficking, synaptic vesicle release |
| Phosphatidylinositol | PI | <5% | Precursor for second messengers (PIP2, IP3, DAG) |
| Cardiolipin | CL | Mitochondria-specific | Mitochondrial membrane structure, oxidative phosphorylation |
Molecular Mechanisms: Phospholipid Regulation of Neuronal Membrane Properties
Structural Foundations of Membrane Fluidity
The amphipathic nature of phospholipids enables spontaneous formation of lipid bilayers in aqueous environments, establishing the foundational structure of neuronal membranes. Membrane fluidity is dynamically regulated by phospholipid composition, particularly the ratio of saturated to unsaturated fatty acids and the incorporation of cholesterol. Phospholipids containing PUFAs increase membrane fluidity and permeability by introducing steric constraints that prevent tight packing of hydrocarbon chains [123]. This enhanced fluidity facilitates lateral diffusion of membrane proteins, including receptors, ion channels, and transporters, thereby influencing synaptic strength and neuronal excitability.
The degree of fatty acid unsaturation in phospholipids significantly alters membrane functionality. Increased unsaturation elevates the number of bisallyic carbons, which exhibit heightened reactivity toward reactive oxygen species (ROS) and oxidative enzymes. This enhanced susceptibility to oxidative modifications is particularly relevant for mitochondrial phospholipids bordering the electron transport chain, the major site of neuronal ROS generation [123]. Oxidative modification of phospholipids can initiate lipid peroxidation cascades, generating reactive aldehydes like malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) that form protein adducts, impair membrane integrity, and contribute to neuronal dysfunction in aging and neurodegeneration [123].
Phospholipid-Dependent Neurotransmission Pathways
Phospholipids and their metabolites serve as potent regulators of synaptic transmission through presynaptic and postsynaptic mechanisms. Lysophosphatidic acid (LPA), a membrane-derived bioactive phospholipid, induces rapid and reversible depression of both excitatory and inhibitory postsynaptic currents via distinct molecular pathways [125]. At glutamatergic synapses, LPA-triggered depression depends on an LPA1/Gαi/o-protein/phospholipase C/myosin light chain kinase cascade at the presynaptic terminal. This signaling pathway increases myosin light chain phosphorylation, triggering actomyosin contraction and reducing the number of synaptic vesicles docked at active zones [125].
At GABAergic synapses, postsynaptic LPA signaling operates through an LPA1/Gα12/13-protein/RhoA/Rho kinase/calcineurin pathway, leading to dephosphorylation and subsequent internalization of the GABAA receptor γ2 subunit [125]. This receptor trafficking occurs through clathrin-mediated endocytosis, effectively reducing the number of functional inhibitory receptors at the synaptic membrane. Endogenous LPA signaling, primarily via LPA1 receptors, mediates activity-dependent inhibitory synaptic plasticity, suggesting that lysophospholipids serve as local messengers that dynamically tune synaptic strength to precedent neuronal activity [125].
Figure 1: LPA Signaling Pathways at Glutamatergic and GABAergic Synapses. LPA activates distinct G-protein coupled pathways at excitatory (red) and inhibitory (green) synapses, leading to synaptic depression through different mechanisms [125].
Experimental Approaches: Methodologies for Investigating Neuronal Phospholipids
Lipidomic Profiling Techniques
Comprehensive lipidomic analysis enables quantitative assessment of phospholipid composition, distribution, and metabolism in neuronal tissues. Modern lipid profiling employs multiple analytical platforms, including:
Nuclear Magnetic Resonance (NMR) Spectroscopy: ³¹P-NMR provides absolute quantification of phospholipid classes without requiring internal standards, making it particularly valuable for assessing total phospholipid concentrations in brain tissue samples [20]. This technique distinguishes different phospholipid headgroups based on their phosphate chemical shifts.
High-Performance Liquid Chromatography with Evaporative Light Scattering Detection (HPLC-ELSD): This separation-based method provides relative quantification of phospholipid species based on retention time and mass detection. While less absolute than NMR, HPLC-ELSD offers higher sensitivity for low-abundance phospholipid species and can be coupled with mass spectrometry for structural identification [20].
Mass Spectrometry-Based Lipidomics: Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) enables high-resolution identification and quantification of individual phospholipid molecular species. This approach can distinguish phospholipids with identical headgroups but different fatty acid compositions, providing detailed insights into membrane fatty acid saturation states and potential lipid peroxidation products [33].
Table 2: Experimental Models for Phospholipid Research in Neurological Function
| Experimental Model | Key Applications | Methodological Considerations |
|---|---|---|
| In vitro neuronal cultures | Investigation of phospholipid trafficking, receptor localization, and synaptic plasticity | Permits controlled manipulation of phospholipid composition; may not fully recapitulate brain complexity |
| Genetic mouse models (e.g., LPA1 receptor knockout) | Elucidation of specific phospholipid signaling pathways in vivo | Reveals behavioral and functional consequences of phospholipid pathway disruption |
| Dietary intervention studies | Assessment of nutritional phospholipid effects on brain composition and function | Requires precise control of dietary formulations and duration; translational relevance to human nutrition |
| Lipidomic profiling of human brain tissue | Identification of phospholipid alterations in neurological disorders | Postmortem interval and tissue processing significantly impact phospholipid integrity |
| Hypoglossal motor system preparation | Electrophysiological analysis of phospholipid effects on synaptic transmission | Well-characterized model for studying excitatory and inhibitory inputs to defined motor neurons |
Electrophysiological Assessment of Synaptic Function
The hypoglossal motor system serves as an exemplary experimental model for investigating phospholipid regulation of synaptic transmission. This preparation enables precise electrophysiological recording of both AMPA receptor-mediated excitatory postsynaptic currents (EPSCs) and GABAA receptor-mediated inhibitory postsynaptic currents (IPSCs) in identified motor neurons [125]. Standard protocols include:
Brainstem Slice Preparation: Acute brainstem slices (250-300 μm thickness) containing the hypoglossal nucleus are prepared from neonatal rodents (P7-P14) using a vibrating microtome. Slices are maintained in oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 25 glucose, saturated with 95% O2/5% CO2 at 32°C [125].
Whole-Cell Patch-Clamp Recording: Hypoglossal motor neurons are visually identified using infrared differential interference contrast (IR-DIC) microscopy. Patch pipettes (3-5 MΩ resistance) are filled with intracellular solution containing (in mM): 140 CsCl, 1 MgCl2, 10 HEPES, 2 Mg-ATP, 0.3 Na-GTP, and 10 EGTA (pH 7.3 with CsOH). Synaptic currents are evoked by electrical stimulation of adjacent synaptic inputs or recorded spontaneously to assess miniature postsynaptic currents [125].
Pharmacological Isolation of Synaptic Currents: Glutamatergic EPSCs are isolated by bath application of GABAA receptor antagonists (bicuculline, 10 μM), while GABAergic IPSCs are isolated using ionotropic glutamate receptor antagonists (CNQX, 20 μM for AMPA receptors; D-AP5, 50 μM for NMDA receptors). LPA effects are tested by bath application (1-5 μM) while monitoring changes in synaptic current amplitude, frequency, and kinetics [125].
The Scientist's Toolkit: Essential Research Reagents and Methodologies
Table 3: Key Research Reagent Solutions for Phospholipid Neuroscience
| Research Reagent | Supplier Examples | Experimental Function | Application Notes |
|---|---|---|---|
| Synthetic phospholipids (DMPC, DPPC, DSPC) | Avanti Polar Lipids, Sigma-Aldrich | Membrane biophysics studies, liposome preparation | Defined acyl chain composition enables structure-function studies |
| Lysophosphatidic acid (LPA) | Cayman Chemical, Tocris | Investigation of lysophospholipid signaling | Multiple LPA species (16:0, 18:0, 18:1) available with different receptor affinities |
| LPA receptor antagonists (Ki16425) | MedChemExpress, Tocris | Pharmacological inhibition of LPA1/LPA3 receptors | Validates specificity of LPA effects in synaptic plasticity experiments |
| Rho kinase inhibitors (Y-27632) | Sigma-Aldrich, Tocris | Inhibition of RhoA/ROCK signaling pathway | Tests involvement of this pathway in LPA-mediated synaptic depression |
| Phospholipase inhibitors (VU0155069) | Cayman Chemical, Abcam | Selective inhibition of phospholipase D1/2 | Elucidates role of phospholipase activity in neurotransmitter release |
| Fluorescent phospholipid analogs (NBD-PC, NBD-PE) | Avanti Polar Lipids, Thermo Fisher | Membrane trafficking and lipid dynamics visualization | Enables live-cell imaging of phospholipid distribution and movement |
| Mass spectrometry lipid standards | Avanti Polar Lipids, Cayman Chemical | Internal standards for lipidomic quantification | Deuterated or odd-chain phospholipids enable absolute quantification |
Pathophysiological Implications and Therapeutic Applications
Phospholipid Alterations in Neurological Disorders
Dysregulation of phospholipid metabolism has been implicated in numerous neurological and psychiatric conditions. In major depression and anxiety disorders, alterations in membrane-forming n-3 PUFAs, glycerolipids, glycerophospholipids, and sphingolipids correlate with behavioral abnormalities and synaptic dysfunction [124]. Preclinical models demonstrate that phospholipid imbalances induce depression- and anxiety-related behaviors, suggesting a causal role in disease pathogenesis rather than merely correlative associations.
Phospholipid composition changes throughout the lifespan, with studies documenting up to a 20% reduction in brain phospholipid levels by age 80, potentially contributing to age-related cognitive decline and memory impairment [20]. The essential nature of mitochondrial phospholipids is demonstrated by the inability of mitochondria to tolerate alterations in these specific lipids, with changes leading to mitochondrial damage and subsequent neural degeneration [123]. Genetic disorders affecting phospholipid biosynthesis, such as Liberfarb syndrome resulting from phosphatidylethanolamine (PE) metabolism defects, provide compelling evidence for the critical role of specific phospholipid classes in neuronal maintenance [126].
Nutritional and Pharmacological Interventions
Dietary supplementation with phospholipids represents a promising therapeutic approach for neurological disorders. Multiple randomized controlled trials have demonstrated that daily intake of 300-600 mg of milk fat globule membrane (MFGM) phospholipids significantly reduces perceived stress and improves cognitive performance under pressure in both children and adults [20]. Maternal supplementation with conjugated linoleic acid (CLA) and docosahexaenoic acid (DHA) in phospholipid form during pregnancy enhances placental transfer of these bioactive lipids, promoting fetal brain development and potentially reducing risk for neurological disorders in offspring [126].
The structural similarity of phospholipids to neuronal membrane components enhances their bioavailability and brain incorporation compared to triglyceride forms. Studies in piglets demonstrate that triglyceride sources of DHA and AA produce more favorable plasma fatty acid profiles and apparent dry matter digestibilities than phospholipid sources from egg, suggesting that the chemical form of dietary lipids significantly influences their metabolic fate and potential neurological benefits [127].
Figure 2: Dietary Phospholipid Intervention Pathway. Dietary phospholipids from various sources influence brain function through sequential processes of bioavailability, brain incorporation, membrane property modification, and functional outcomes [20] [126].
Phospholipids represent critical determinants of neuronal membrane fluidity, synaptic efficacy, and overall brain function. Their dual roles as structural membrane components and signaling precursors position them as essential mediators of brain health across the lifespan. Evidence from basic science, clinical studies, and nutritional interventions consistently demonstrates that phospholipid composition directly influences cognitive performance, stress resilience, and vulnerability to neurological disorders.
Future research should prioritize several key areas: (1) elucidation of cell-type specific phospholipid metabolism in the brain using single-cell lipidomics; (2) development of targeted phospholipid-based therapeutics for neurodegenerative conditions; and (3) personalized nutrition approaches based on genetic polymorphisms in phospholipid metabolic pathways. The integration of advanced lipidomic methodologies with neurophysiological techniques will further unravel the complex relationships between phospholipid structure, neuronal function, and cognitive outcomes, opening new avenues for maintaining neurological health through precision lipid interventions.
Dietary lipids are fundamental to human health, serving as essential structural components of cellular membranes and as potent metabolic fuels. Within this broad category, phospholipids and medium-chain triglycerides (MCTs) represent two distinct classes with unique chemical structures and physiological pathways. Phospholipids are amphipathic molecules characterized by a glycerol backbone esterified to two fatty acid chains and a phosphate-linked polar head group [21] [22]. This structure enables them to form the fundamental bilayer architecture of all cellular membranes, providing not just structural integrity but also functional platforms for cellular signaling [128] [21]. In contrast, MCTs are composed of a glycerol backbone esterified to three medium-chain fatty acids (typically 8-12 carbons in length) [129]. Their relatively shorter chain length confers distinct metabolic properties, allowing them to be rapidly absorbed and hydrolyzed, then transported directly to the liver via the portal circulation where they serve as a preferential substrate for ketogenesis [130] [129]. This review synthesizes evidence from clinical and preclinical studies examining the therapeutic potential of phospholipid supplementation and MCT interventions, framed within the broader context of dietary lipid research.
Phospholipid Supplementation: Mechanisms and Therapeutic Applications
Molecular Mechanisms of Action
The therapeutic efficacy of dietary phospholipids stems from their direct incorporation into cellular membranes and subsequent influence on membrane dynamics and signaling. Upon ingestion, phospholipids are efficiently absorbed (>90%), with a significant portion incorporated intact into circulating lipoproteins for delivery to various tissues [128] [131]. The incorporated phospholipids then modulate membrane fluidity, microdomain organization, and lipid raft composition, thereby influencing the activity of membrane-bound receptors and transporters [128]. Furthermore, the fatty acid composition of the supplemental phospholipids determines the profile of lipid second messengers, such as eicosanoids, derived from membrane phospholipid pools [128]. For instance, phospholipids rich in arachidonic acid may promote the synthesis of pro-inflammatory eicosanoids, while those containing eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) support anti-inflammatory signaling cascades [128].
Key Clinical and Preclinical Findings
Table 1: Evidence for Phospholipid Health Benefits Across Physiological Systems
| Physiological System | Reported Benefits | Study Type | Key Findings | Proposed Mechanism |
|---|---|---|---|---|
| Cognitive Health | Support for memory, focus, and stress resilience [20] | Human Randomized Controlled Trials | Daily intake of 300-600 mg of milk fat globule membrane (MFGM) phospholipids improved cognitive performance under pressure [20]. | Incorporation into neuronal membranes, support of neurotransmitter activity (e.g., acetylcholine synthesis) [20]. |
| Mitochondrial Function & Fatigue | Reduction of fatigue in aging and chronic illnesses [131] | Human Clinical Trials | Membrane Lipid Replacement (MLR) with phospholipids (e.g., NTFactor) restored mitochondrial function and reduced fatigue [131]. | Replacement of oxidized membrane lipids in mitochondria, improving membrane integrity and ATP production efficiency [131]. |
| Metabolic & Cardiovascular Health | Improved plasma lipid profiles [131] | Human Clinical Trials | Phosphatidylinositol supplementation (≥5 g/day) increased HDL-cholesterol and apolipoprotein A1, while reducing triglycerides [131]. | Enhanced reverse cholesterol transport and modulation of lipoprotein metabolism [128] [131]. |
| Inflammatory Pathways | Modulation of inflammation [128] | Preclinical (in vitro and animal studies) | Dietary phospholipids from marine sources (with EPA/DHA) reduced synthesis of pro-inflammatory eicosanoids [128]. | Alteration of membrane fatty acid composition, leading to production of less inflammatory eicosanoid precursors [128]. |
Experimental Protocols for Phospholipid Research
Protocol for Assessing Membrane Incorporation of Dietary Phospholipids: A standard methodology to evaluate the integration and effects of dietary phospholipids involves in vivo supplementation followed by membrane analysis [128] [131]. Subjects (animal or human) receive a defined daily dose of phospholipids (e.g., 1-5 grams) from a specific source (soy, egg, marine) for a predetermined period (weeks to months). Blood samples are collected pre- and post-intervention. Peripheral blood mononuclear cells (PBMCs) or other accessible cells are isolated via density gradient centrifugation. Lipids are extracted from these cells using a chloroform-methanol mixture (e.g., Folch method). The phospholipid fraction is separated by thin-layer chromatography or high-performance liquid chromatography (HPLC-ELSD) [20]. The fatty acid composition of specific phospholipid classes (e.g., phosphatidylcholine) is then analyzed by gas chromatography to quantify the incorporation of fatty acids from the supplement into cellular membranes [128].
MCT Interventions: Metabolic Regulation and Neurological Applications
Unique Metabolic Pathways of MCTs
The metabolic fate of MCTs differs fundamentally from that of long-chain triglycerides (LCTs). While LCTs require packaging into chylomicrons and transit through the lymphatic system, MCTs are rapidly hydrolyzed in the intestine, and the resulting medium-chain fatty acids (MCFAs) are absorbed directly into the portal vein [129] [132]. This bypasses the carnitine palmitoyltransferase (CPT-1) dependent transport system required for long-chain fatty acid entry into mitochondria [132]. Consequently, MCFAs are rapidly transported to the liver and undergo preferential beta-oxidation, leading to significant production of the ketone bodies beta-hydroxybutyrate (BHB) and acetoacetate (AcAc) [130] [129]. These ketones are then released into the bloodstream, providing an alternative energy substrate for peripheral tissues, particularly the brain [130].
Diagram 1: MCT Metabolic Pathway to Brain Energy. This pathway illustrates how MCTs are rapidly converted to ketones, providing an alternative fuel for the brain.
Key Clinical and Preclinical Findings
Table 2: Evidence for MCT Health Benefits Across Physiological Systems
| Application Area | Reported Benefits | Study Type | Key Findings | Proposed Mechanism |
|---|---|---|---|---|
| Neurological Health (Alzheimer's Disease) | Stabilization or improvement of cognitive function [130] | 6-month RCT with open-label extension [130] | 80% of AD subjects (n=20) on 42 g/day MCT (avg. tolerated: 25.2 g/day) showed stabilized or improved cognition (MMSE, MoCA). Longer exposure (>9 months) correlated with better outcomes [130]. | Provision of ketones as an alternative cerebral fuel, bypassing cerebral glucose hypometabolism [130]. |
| Energy Homeostasis & Body Composition | Increased energy expenditure, satiety, modest weight loss [129] | Human Intervention Studies | MCT intake (10-20 g/day) was associated with increased thermogenesis and fat oxidation compared to LCTs [129]. | Rapid beta-oxidation and hepatic conversion of MCFAs, leading to increased energy expenditure [129] [132]. |
| Glucose Metabolism | Improved glucose homeostasis [129] | Human and Preclinical Studies | MCT supplementation has been linked to improved insulin sensitivity and glycemic control [129]. | Modulation of hepatic glucose production and enhancement of peripheral glucose uptake, potentially via ketone signaling [129]. |
| Exercise Performance | Minimal to no ergogenic effects [132] | Systematic Review of Human Studies | Most studies reported no improvement in endurance performance or substrate utilization (e.g., respiratory exchange ratio, fat oxidation) despite elevated ketone levels [132]. | Inability to utilize MCT-induced ketones as a primary energy source during acute, high-intensity exercise; potential gastrointestinal distress at high doses (>30 g) [132]. |
Experimental Protocols for MCT Research
Protocol for Evaluating Cognitive Outcomes in Alzheimer's Disease: A robust double-blind, randomized, placebo-controlled crossover design with an open-label extension can be employed to assess the efficacy of MCT oil on cognition [130]. Participants with mild-to-moderate Alzheimer's disease (diagnosed per NINCDS-ADRDA criteria) are randomized to receive either MCT oil or a placebo (e.g., long-chain triglyceride oil) for an initial period (e.g., 3-5 months), followed by a washout period and crossover to the alternate treatment. A subsequent open-label phase allows all participants to receive MCT oil for an extended period (e.g., 6 months). The MCT dose is titrated to a target (e.g., 42 g/day) or the maximum tolerated dose to minimize gastrointestinal adverse effects. Cognitive function is assessed using standardized tools like the Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and computer-based cognitive assessment systems (e.g., Cognigram) at baseline and regular intervals throughout the study [130]. Blood ketone levels (BHB) are measured to confirm biochemical efficacy and correlate with cognitive outcomes.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagents for Phospholipid and MCT Investigations
| Reagent / Material | Function / Application | Example Use Cases |
|---|---|---|
| Source-Specific Phospholipids (Soy, Egg, Marine, Milk) [128] | To investigate the impact of phospholipid headgroup and fatty acid composition on biological outcomes. | Comparing the anti-inflammatory effects of marine-derived (EPA/DHA) vs. soy-derived (linoleic acid) phospholipids in cell culture or animal models of inflammation [128]. |
| MCT Oil / Purified MCFAs (C8, C10, C12) [130] [129] | To study the metabolic and cognitive effects of specific medium-chain fatty acids. | Examining the ketogenic potential and tolerability of caprylic acid (C8) vs. capric acid (C10) in human clinical trials [130] [129]. |
| Liposomal Formulation Kits [20] [22] | To create lipid bilayers for drug delivery or membrane studies. | Developing liposomal carriers for enhanced bioavailability of therapeutic agents or creating model membranes for biophysical studies [22]. |
| Ketone Body Assays (BHB, AcAc) [130] [132] | To quantify ketone levels in blood, serum, or cell culture media as a marker of MCT metabolism. | Monitoring ketosis in human subjects supplemented with MCT oil and correlating BHB levels with cognitive or metabolic parameters [130]. |
| Gas Chromatography (GC) System [128] | To analyze the fatty acid profile of phospholipids in tissues, cells, or supplements. | Determining the extent of incorporation of supplemental phospholipid fatty acids into cellular membranes in animal studies [128]. |
| 31P-NMR Spectroscopy / HPLC-ELSD [20] | For the identification and quantification of individual phospholipid classes in complex mixtures. | Characterizing the phospholipid composition of a novel supplement or a biological sample [20]. |
Diagram 2: Generalized Experimental Workflow. This chart outlines a logical pathway for designing research on phospholipids and MCTs, from hypothesis to analysis.
Phospholipid supplementation and MCT interventions offer distinct, evidence-based approaches to modulating human health by targeting fundamental cellular and metabolic processes. Phospholipids function primarily as membrane-modifying agents, with demonstrated benefits for cognitive function, fatigue reduction, and metabolic parameters, largely through their direct incorporation into cellular structures [128] [20] [131]. In contrast, MCTs act primarily as metabolic fuels and ketone precursors, showing promise for neurological conditions like Alzheimer's disease by providing the brain with an alternative energy source [130] [129]. The efficacy of both interventions is critically dependent on factors such as source, composition, and dosage. Future research should focus on elucidating detailed molecular mechanisms, validating these findings in larger and more diverse human populations, and standardizing therapeutic protocols to fully realize the potential of these versatile dietary lipids in clinical and nutraceutical applications.
Conclusion
The distinct chemical structures of triglycerides and phospholipids dictate their unique and essential roles in human physiology and disease. While triglycerides serve as paramount energy reservoirs, their structure and fatty acid composition, particularly medium-chain triglycerides (MCTs), directly influence metabolic pathways with therapeutic potential for conditions like epilepsy and glucose intolerance. Phospholipids, by virtue of their amphipathic nature, are indispensable for cellular integrity, signaling, and as enabling tools in drug delivery. The convergence of research on these lipid classes highlights critical interplay in metabolic health, with phospholipids modulating the effects of triglycerides via lipoprotein dynamics and hepatic function. Future directions for biomedical research include the rational design of structured triglycerides with defined nutritional and pharmaceutical properties, the exploitation of phospholipid diversity for targeted nanomedicine, and deeper exploration of how dietary lipid structures influence the gut-brain axis and microbiome. For drug development, this underscores the opportunity to leverage these natural biomolecules as both active ingredients and advanced delivery systems to improve therapeutic outcomes.
References
- 1. The Role of Glycerol and Its Derivatives in the Biochemistry ... [https://www.mdpi.com/2073-4344/11/1/86]
- 2. Glycerol Backbone - (Organic Chemistry) [https://fiveable.me/key-terms/organic-chem/glycerol-backbone]
- 3. Glycerolipids: Structure, Synthesis, Function and Analytical ... [https://www.creative-proteomics.com/resource/glycerolipids-structure-synthesis-function-analysis.htm]
- 4. Current Research in Phospholipids and Their Use in Drug ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7766331/]
- 5. Glycerophospholipids in ALS: insights into disease ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12297743/]
- 6. Glycerolipids in photosynthesis: Composition, synthesis ... [https://www.sciencedirect.com/science/article/pii/S0005272813001631]
- 7. Structures, functions, and syntheses of glycero ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10881803/]
- 8. Polymorphic Structures and Transitions of Triglycerides ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12497477/]
- 9. Review of Eukaryote Cellular Membrane Lipid ... [https://www.mdpi.com/1422-0067/24/21/15693?ref=PDF]
- 10. A sustainable and regioselective synthesis of Hemi-bis ... [https://www.sciencedirect.com/science/article/pii/S2589004223011525]
- 11. Triglyceride [https://en.wikipedia.org/wiki/Triglyceride]
- 12. Triglyceride - an overview | ScienceDirect Topics [https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/triglyceride]
- 13. between Structural and phospholipids from... comparison triglycerides [https://link.springer.com/article/10.1007/BF02531178]
- 14. Hypertriglyceridemia Pipeline Market Research 2025: [https://www.globenewswire.com/news-release/2025/03/04/3036731/28124/en/Hypertriglyceridemia-Pipeline-Market-Research-2025-Comprehensive-Review-on-18-Companies-and-20-Pipeline-Drugs.html]
- 15. Triglyceride - an overview | ScienceDirect Topics [https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/triglyceride]
- 16. Olezarsen reduces levels of the blood fat, triglycerides, in ... [https://www.escardio.org/The-ESC/Press-Office/Press-releases/Olezarsen-reduces-levels-of-the-blood-fat-triglycerides-in-patients-at-high-cardiovascular-risk]
- 17. Triglyceride Structure [https://byjus.com/chemistry/triglyceride-structure/]
- 18. 26.8: Triglycerides [https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/26%3A_Biochemistry/26.08%3A_Triglycerides]
- 19. 6.1 Triglycerides and Fatty Acids – Nutrition and Physical ... [https://pressbooks.calstate.edu/nutritionandfitness/chapter/6-1-triglycerides-and-fatty-acids/]
- 20. Phospholipid [https://en.wikipedia.org/wiki/Phospholipid]
- 21. 3.5: Lipid Molecules - Phospholipids [https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/General_Biology_(Boundless)/03%3A_Biological_Macromolecules/3.05%3A_Lipid_Molecules_-_Phospholipids]
- 22. Phospholipids: Structure, Functions, and Applications [https://www.creative-proteomics.com/resource/phospholipids-structure-functions-and-applications.htm]
- 23. Lipids in Clinical Nutrition and Health: Narrative Review ... [https://www.mdpi.com/2304-8158/14/3/473]
- 24. Webinar July 2025 - Prof. Blume [https://www.phospholipid-research-center.com/event/webinar-july-2025-prof-blume/]
- 25. The 8th International Symposium on Phospholipids in ... [https://www.sciencedirect.com/science/article/pii/S0928098725001253]
- 26. Recent advances in DHA-containing phospholipids (PL ... [https://www.sciencedirect.com/science/article/abs/pii/S0963996925011408]
- 27. 5.3 Three Classes of Lipids – Introduction to Nutrition and ... [https://mtsu.pressbooks.pub/nutrition/chapter/5b-lipid-types-structures/]
- 28. Biochemistry, Lipids - StatPearls - NCBI Bookshelf - NIH [https://www.ncbi.nlm.nih.gov/books/NBK525952/]
- 29. Phosphatidylcholine [https://en.wikipedia.org/wiki/Phosphatidylcholine]
- 30. Phosphatidylethanolamine [https://en.wikipedia.org/wiki/Phosphatidylethanolamine]
- 31. Sphingomyelin [https://en.wikipedia.org/wiki/Sphingomyelin]
- 32. Phosphatidylserine [https://en.wikipedia.org/wiki/Phosphatidylserine]
- 33. Lipidome changes due to improved dietary fat quality ... [https://www.nature.com/articles/s41591-024-03124-1]
- 34. Dietary phospholipids improve growth performance and ... [https://www.sciencedirect.com/science/article/pii/S2352513423001060]
- 35. Interdigitation between Triglycerides and Lipids Modulates ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5390054/]
- 36. Lipid bilayer [https://en.wikipedia.org/wiki/Lipid_bilayer]
- 37. Structure of the Plasma Membrane - The Cell - NCBI Bookshelf [https://www.ncbi.nlm.nih.gov/books/NBK9898/]
- 38. Late-Breaking Science Highlights New Treatments For ... [https://www.acc.org/latest-in-cardiology/articles/2025/11/03/16/19/sat-940am-hypertriglyceridemia-aha-2025]
- 39. Overview ‹ TESSERAE: Self-Assembling Space Architecture [https://www.media.mit.edu/projects/tesserae-self-assembling-space-architecture/overview/]
- 40. Nanoscale Spatial Control over the Self-Assembly of Small ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12026908/]
- 41. B. Lipids: Membranes and Fats [https://chem.libretexts.org/Courses/Saint_Marys_College_Notre_Dame_IN/CHEM_342%3A_Bio-inorganic_Chemistry/Readings/Chapter_5%3A_Biological_Molecules/Structures_of_Biological_Molecules/B._Lipids%3A_Membranes_and_Fats]
- 42. The role of phospholipids in the biological activity and ... [https://www.sciencedirect.com/science/article/pii/S0167488913002085]
- 43. Advances in Oral Drug Delivery Systems for Natural ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12655038/]
- 44. Comparison of bioavailability of krill oil versus fish oil and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4559234/]
- 45. Metabolic Effects of n-3 PUFA as Phospholipids Are Superior ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3372498/]
- 46. Food Structure Modulates the Bioavailability of Triglycerides and... [https://pubmed.ncbi.nlm.nih.gov/32966703/]
- 47. Light-scattering detection of phospholipids resolved by HPLC [https://pubmed.ncbi.nlm.nih.gov/14584608/]
- 48. Measurement of Ether Phospholipids in Human Plasma ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4958133/]
- 49. Polymorphic phase transitions in triglycerides and their ... [https://www.sciencedirect.com/science/article/pii/S0001868623002385]
- 50. Multiscale analysis of triglycerides using X-ray scattering [https://pubmed.ncbi.nlm.nih.gov/38887036/]
- 51. a comprehensive time-resolved X-ray scattering study [https://pubs.rsc.org/en/content/articlelanding/2024/ce/d4ce00800f]
- 52. Introduction to Lipids and Lipoproteins - Endotext - NCBI - NIH [https://www.ncbi.nlm.nih.gov/books/NBK305896/]
- 53. Supplementation of Regular Diet With Medium-Chain ... [https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2022.934497/full]
- 54. Medium-chain triglycerides improve cognition and systemic ... [https://pubmed.ncbi.nlm.nih.gov/40794774/]
- 55. Effect of Synthetic Dietary Triglycerides: A Novel Research ... [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0001681]
- 56. The structure of triglycerides impacts the digestibility and ... [https://pubmed.ncbi.nlm.nih.gov/36996650/]
- 57. Dietary Triacylglycerol Structure and Its Role in Infant ... [https://www.sciencedirect.com/science/article/pii/S2161831322005907]
- 58. Research progress, challenges and perspectives of ... [https://pubmed.ncbi.nlm.nih.gov/38362962/]
- 59. The Role of Phospholipid Alterations in Mitochondrial and ... [https://www.mdpi.com/1422-0067/25/9/4645]
- 60. Phospholipid Metabolism - an overview [https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/phospholipid-metabolism]
- 61. A Systematic Review of Advances in Plant-Based ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12472430/]
- 62. Phytosomes: A promising nanocarrier system for enhanced ... [https://www.sciencedirect.com/science/article/pii/S2667031325000521]
- 63. Pharmacological mechanisms and drug delivery systems ... [https://pubmed.ncbi.nlm.nih.gov/40414584/]
- 64. Advances and Emerging Trends in Drug Delivery Systems [https://juniperpublishers.com/jpcr/JPCR.MS.ID.555802.php]
- 65. Long-acting drug delivery systems: Current landscape and ... [https://www.sciencedirect.com/science/article/pii/S1359644625001606]
- 66. | Cell Membrane, Lipid Bilayer & Fatty Acids | Britannica Phospholipid [https://www.britannica.com/science/phospholipid]
- 67. 26.9: Phospholipids - Chemistry LibreTexts [https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/26%3A_Biochemistry/26.09%3A_Phospholipids]
- 68. sciencedirect.com/topics/ food - science / phospholipids [https://www.sciencedirect.com/topics/food-science/phospholipids]
- 69. Advances in emulsion stability: A review on mechanisms ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12311586/]
- 70. Tuning interfacial properties of phospholipid stabilised oil– ... [https://www.sciencedirect.com/science/article/pii/S002197972500267X]
- 71. Addition of Phospholipids Improved the Physical Stability ... [https://www.mdpi.com/2304-8158/14/3/375]
- 72. What is Phospholipids ? Uses, How It Works & Top Companies ( 2025 ) [https://www.linkedin.com/pulse/what-phospholipids-uses-how-works-top-companies-8a5ee]
- 73. Controlled lipid digestion in the development of functional ... [https://www.sciencedirect.com/science/article/pii/S0308814624038019]
- 74. Tailoring plant lipid composition: designer oilseeds come ... [https://www.sciencedirect.com/science/article/abs/pii/S1369526610000154]
- 75. Tailoring and optimizing fatty acid production by ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9632096/]
- 76. Triglycerides as Novel Phase-Change Materials: A Review ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7730147/]
- 77. Emerging applications of stimulated Raman scattering ... [https://pubs.rsc.org/en/content/articlehtml/2025/cs/d5cs00748h]
- 78. LipoGalen™ – Our new family of functional lipid-based ... [https://www.ioioleo.de/en/news/lipogalen-our-new-family-of-functional-lipid-based-excipients/]
- 79. Development and application of a novel tracer system for ... [https://pubmed.ncbi.nlm.nih.gov/41061904/]
- 80. Development and application of a novel tracer system for ... [https://www.sciencedirect.com/science/article/pii/S0939641125002668]
- 81. Triglyceride, fatty acid profile and antioxidant ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6408781/]
- 82. Phospholipids at the Interface: Current Trends and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3709755/]
- 83. The structure of triglycerides impacts the digestibility and ... [https://www.sciencedirect.com/science/article/abs/pii/S0308814623005642]
- 84. Interfacial Behaviour of Egg Yolk Extracts | Food Biophysics [https://link.springer.com/article/10.1007/s11483-019-09572-4]
- 85. Inter-relationships between composition, physicochemical ... [https://www.sciencedirect.com/science/article/pii/S0924224421001357]
- 86. Ultrasound-assisted pH-shifting treatment: Effects on egg ... [https://www.sciencedirect.com/science/article/abs/pii/S0308814625014232]
- 87. Role of lysophospholipids on the interfacial and liquid film ... [https://www.sciencedirect.com/science/article/abs/pii/S0268005X19308045]
- 88. Structure, Biological Functions, Separation, Properties, and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10935281/]
- 89. Phospholipids in Milk Fat: Composition, Biological and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3588016/]
- 90. Milk phospholipid-coated lipid droplets modulate the infant gut ... [https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-025-02106-w]
- 91. Milk fat globule membrane supplementation in formula ... [https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2025.1632519/full]
- 92. Effect of Technological Process and Temperature on ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12346082/]
- 93. Formulations based on milk fat globule membrane as ... [https://link.springer.com/article/10.1007/s00217-025-04812-z]
- 94. Enhancing Drug Solubility, Bioavailability, and Targeted ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11510236/]
- 95. Solubilization techniques used for poorly water-soluble drugs [https://pmc.ncbi.nlm.nih.gov/articles/PMC11628819/]
- 96. 22/04/ 2025 - New Whitepaper " Bioavailability Enhancement..." [https://www.aenova-group.com/en/news-events/news/22-04-2025-new-whitepaper-on-bioavailability-enhancement-strategies-for-poorly-soluble-drugs]
- 97. 2.7: Lipids - Triglycerides, Phospholipids and Sterols [https://med.libretexts.org/Courses/Kansas_State_University/FNDH_400%3A_Human_Nutrition_(Lindshield)/02%3A_Macronutrient_Structures/2.07%3A_Lipids_-_Triglycerides_Phospholipids_and_Sterols]
- 98. Review Oral lipid-based drug delivery systems – an overview [https://www.sciencedirect.com/science/article/pii/S2211383513000919]
- 99. Advances in nanoparticles in targeted drug delivery–A review [https://www.sciencedirect.com/science/article/pii/S2666845925001163]
- 100. Engineering precision nanoparticles for drug delivery [https://www.nature.com/articles/s41573-020-0090-8]
- 101. Phospholipids vs. Triglycerides: The Differences [https://iwilife.com/blogs/news/phospholipid-vs-triglyceride?srsltid=AfmBOopZQxToa5AcNjbNzz0mQnPUYyF6JIE8lwdZsEObC4JZDLEbc1bW]
- 102. Structural and signaling role of lipids in plasma membrane ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7182362/]
- 103. Metabolism of Triglyceride-Rich Lipoproteins - NCBI - NIH [https://www.ncbi.nlm.nih.gov/books/NBK584295/]
- 104. Triglyceride cycling enables modification of stored fatty acids [https://www.nature.com/articles/s42255-023-00769-z]
- 105. Membrane Lipids and Cell Signaling - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC5776726/]
- 106. Lipid-based signaling pathways | GeneGlobe [https://geneglobe.qiagen.com/us/knowledge/pathways/general-signal-transduction-pathways/lipid-based-signaling]
- 107. Phospholipids vs. Triglycerides: The Differences [https://iwilife.com/blogs/news/phospholipid-vs-triglyceride?srsltid=AfmBOorqCfoJWw_ifOrmcTKyT-Mx9ECJN8i591fH9Mtuuqpod-hQO8Gn]
- 108. Name A Structural Difference Between Triglycerides And ... [https://dev.housing.arizona.edu/name-a-structural-difference-between-triglycerides-and-phospholipids]
- 109. Triglyceride Metabolism: Structure, Regulation, and Role in ... [https://www.metwarebio.com/triglyceride-metabolism-structure-regulation-and-role-in-metabolic-diseases/]
- 110. Compare and contrast the structures of phospholipids and ... [https://www.pearson.com/channels/anp/asset/7a4440b0/compare-and-contrast-the-structures-of-phospholipids-and-triglycerides]
- 111. Phospholipids and Cholesterol Determine Molecular ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12152213/]
- 112. Lipid-based delivery systems and intestinal lymphatic drug ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7103284/]
- 113. Lymphatic vessel: Origin, heterogeneity, biological ... [https://www.nature.com/articles/s41392-023-01723-x]
- 114. Lymphatic and portal vein absorption of organochlorine ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2643918/]
- 115. Absorption and distribution of dietary fatty acids from ... [https://pubmed.ncbi.nlm.nih.gov/11755040/]
- 116. Positional distribution of fatty acids in dietary triglycerides [https://www.sciencedirect.com/science/article/pii/S000291652318600X]
- 117. An update on oral drug delivery via intestinal lymphatic ... [https://www.sciencedirect.com/science/article/pii/S2211383521000344]
- 118. The critical role of phosphatidylcholine and ... [https://www.sciencedirect.com/science/article/pii/S0005273617301220]
- 119. Redefining the association of triglycerides with all-cause ... [https://www.sciencedirect.com/science/article/pii/S2666606525002287]
- 120. A Review of Molecular and Metabolic Pathways Involved [https://pmc.ncbi.nlm.nih.gov/articles/PMC8960636/]
- 121. Multi-omics analysis reveals metabolic regulation of ... [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0332177]
- 122. Comprehensive analysis of risk factors and metabolic profiling ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12611969/]
- 123. The Role of Phospholipid Alterations in Mitochondrial and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11083216/]
- 124. Brain membrane lipids in major depression and anxiety ... [https://www.sciencedirect.com/science/article/pii/S1388198114002662]
- 125. Membrane-Derived Phospholipids Control Synaptic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4440815/]
- 126. Editorial: Phospholipids and sphingolipids in nutrition, ... [https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2023.1153138/full]
- 127. Comparison of Triglycerides and Phospholipids as ... [https://www.sciencedirect.com/science/article/pii/S0022316622155100]
- 128. Health effects of dietary phospholipids - PMC - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC3316137/]
- 129. Dietary medium-chain triacylglycerols in metabolic regulation [https://www.sciencedirect.com/science/article/pii/S1043276025002164]
- 130. Use of medium chain triglyceride (MCT) oil in subjects with ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8919247/]
- 131. Clinical Uses of Membrane Lipid Replacement ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6941554/]
- 132. The Effects of Medium-Chain Triglyceride Oil ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9579472/]