Beyond Browning: Decoding the Chemistry of Non-Enzymatic Browning and the Maillard Reaction for Biomedical Research
This article provides a comprehensive analysis of the complex chemistry of non-enzymatic browning, with a focus on the Maillard reaction, tailored for researchers, scientists, and drug development professionals.
Beyond Browning: Decoding the Chemistry of Non-Enzymatic Browning and the Maillard Reaction for Biomedical Research
Abstract
This article provides a comprehensive analysis of the complex chemistry of non-enzymatic browning, with a focus on the Maillard reaction, tailored for researchers, scientists, and drug development professionals. It covers the foundational mechanisms, from initial Schiff base formation to advanced glycation end-products (AGEs), and explores advanced methodological approaches like FT-ICR-MS for characterizing reaction products. The content details strategies to control reaction parameters and troubleshoot the formation of detrimental compounds like acrylamide. A comparative analysis evaluates the reactivity of different precursors and the interplay with other browning pathways. The review concludes by synthesizing the implications of these reactions in human health, particularly in diabetes and aging, and their relevance to drug stability and development.
The Core Chemistry: Unraveling the Mechanisms of Non-Enzymatic Browning
Non-enzymatic browning (NEB) represents a group of complex chemical reactions that profoundly impact the quality, safety, and nutritional value of processed foods, pharmaceuticals, and biological systems. These reactions are distinct from enzymatic browning, which involves polyphenol oxidase acting on phenolic compounds. Instead, NEB occurs without enzyme catalysis and encompasses three primary pathways: the Maillard reaction, caramelization, and ascorbic acid oxidation [1] [2]. For researchers and drug development professionals, understanding these pathways is crucial for controlling product stability, shelf-life, and preventing the formation of potentially harmful compounds.
The Maillard reaction, first described by Louis Camille Maillard in 1912, involves reactions between nucleophilic amino groups (from amino acids, peptides, or proteins) and carbonyl groups (primarily from reducing sugars) [2]. Caramelization refers to the thermal degradation of sugars in the absence of amino compounds, while ascorbic acid oxidation involves the degradation of vitamin C, which can generate brown pigments [3] [4]. These reactions not only govern the sensory attributes of foods and pharmaceuticals but also produce advanced glycation end products (AGEs) with potential implications for human health and drug stability.
This technical guide provides an in-depth analysis of the core NEB pathways, emphasizing reaction mechanisms, kinetics, analytical methodologies, and experimental approaches relevant to scientific research and industrial applications.
Fundamental Mechanisms of Non-Enzymatic Browning Pathways
The Maillard Reaction
The Maillard reaction proceeds through three progressive stages, generating a complex array of flavor compounds, pigments, and macromolecular structures [1] [2].
Initial Stage (Early Maillard Reaction): The reaction begins with a nucleophilic addition, where the carbonyl group of a reducing sugar (e.g., glucose, fructose) condenses with the free amino group of an amino acid, peptide, or protein to form an unstable N-glycosylamine. This compound rapidly undergoes an Amadori rearrangement to form a more stable Amadori rearrangement product (ARP) (for aldoses) or a Heyns product (for ketoses) [1] [2]. This stage is reversible, and no browning is visually apparent.
Intermediate Stage: Under appropriate pH and temperature conditions, the ARP degrades through multiple pathways. At pH ≤ 7, 1,2-enolization dominates, leading to the formation of furfurals (e.g., hydroxymethylfurfural (HMF) from hexoses) [1]. At pH > 7, 2,3-enolization occurs, producing reductones and fission products like α-dicarbonyl compounds (glyoxal, methylglyoxal, diacetyl) [1] [4]. These highly reactive α-dicarbonyls are pivotal intermediates. They can further undergo Strecker degradation when reacting with amino acids, producing Strecker aldehydes with one less carbon atom, ammonia, and α-aminoketones [1]. These reactions generate characteristic aromas and flavors.
Final Stage: In the terminal phase, condensation, polymerization, and cyclization reactions occur between the various intermediates (furan derivatives, aldehydes, aminoketones). This complex network ultimately leads to the formation of heterogeneous, high-molecular-weight, brown-colored nitrogenous polymers known as melanoidins [1] [2].
The following diagram illustrates the sequential stages and key intermediates of the Maillard reaction pathway:
Caramelization
Caramelization is the pyrolysis of sugars, typically occurring at high temperatures (above 120-150°C) and in the absence of amino compounds [1]. The process initiates with the enolization of sugar molecules, followed by dehydration reactions that lead to the formation of anhydro-sugars and furan derivatives such as HMF (from hexoses) or furfural (from pentoses) [1]. Subsequent condensation and polymerization of these fragments yield a complex mixture of high-molecular-weight compounds collectively known as caramelans, which impart the characteristic deep brown color and caramel flavor [1]. While both caramelization and the Maillard reaction can produce furans and brown pigments, caramelization does not involve nitrogenous compounds, distinguishing its reaction pathway and end-products.
Ascorbic Acid Oxidation
Ascorbic acid (ASA) degradation is a significant NEB pathway, particularly in acidic and fruit-based products like orange juice [3] [4]. Ascorbic acid can oxidize to dehydroascorbic acid, which subsequently undergoes hydrolytic ring opening and further degradation. These reactions generate reactive carbonyl species (RCS), including xylosone, furfural, and α-dicarbonyls [3] [4]. These carbonyl intermediates can then polymerize to form brown pigments or, in the presence of amino acids, enter the Maillard reaction pathway, thereby acting as both a browning precursor and a participant in parallel reaction networks [4].
Table 1: Comparative Overview of Primary Non-Enzymatic Browning Pathways
| Feature | Maillard Reaction | Caramelization | Ascorbic Acid Oxidation |
|---|---|---|---|
| Primary Reactants | Carbonyl compounds (reducing sugars) + Amino compounds (amino acids, proteins) | Sugars (reducing or non-reducing) | Ascorbic Acid |
| Key Initiating Step | Nucleophilic addition & Amadori/Heyns rearrangement | Enolization & dehydration | Oxidation & ring opening |
| Nitrogen Involvement | Essential | Absent | Can involve amino acids secondarily |
| Characteristic Intermediates | Strecker aldehydes, α-dicarbonyls, furfurals | Furan derivatives, anhydro-sugars | Xylosone, furfural, α-dicarbonyls |
| Primary End Products | Melanoidins (nitrogenous brown polymers) | Caramelan (non-nitrogenous polymers) | Brown pigments |
| Typical Reaction Conditions | Wide range of temperatures and pH | High temperatures (>~120°C), various pH | Prevalent in acidic media (e.g., fruit juices) |
Analytical Methodologies for Investigating NEB
A multifaceted analytical approach is required to monitor NEB progress, identify intermediates, and quantify end-products. The choice of method depends on the specific research goals and the complexity of the sample matrix [1].
Spectrophotometric Techniques
Spectrophotometry provides a rapid, low-cost means for relative comparison of NEB progression.
- Browning Intensity (A420 nm): Absorbance at 420 nm measures brown pigment formation, indicative of the final stage products like melanoidins [1] [5].
- Intermediate Products (A294 nm): Absorbance at 294 nm monitors the formation of colorless intermediate products, such as furfurals and other chromophores [6] [5].
Chromatographic and Mass Spectrometric Techniques
For specific identification and quantification, chromatographic methods coupled with various detectors are employed.
- High-Performance Liquid Chromatography (HPLC): Used to quantify specific markers like 5-hydroxymethylfurfural (HMF), furosine (a marker for ARP), and N-ε-carboxymethyllysine (CML) [1] [7]. It can also track the depletion of reactants like ascorbic acid and amino acids [5].
- Liquid Chromatography-Mass Spectrometry (LC-MS): Essential for sensitive and specific identification and quantification of non-volatile MRPs, including advanced glycation end-products (AGEs) like CML and CEL [1] [7].
- Gas Chromatography-Mass Spectrometry (GC-MS): Ideal for profiling volatile MRPs and caramelization products, such as pyrazines, furans, and Strecker aldehydes [1] [6].
Advanced Structural Analysis
- Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR-MS): This ultra-high-resolution mass spectrometry technique is powerful for non-targeted analysis of complex NEB reaction systems. It can assign molecular formulae to thousands of reaction products simultaneously, providing a comprehensive view of the "chemodiversity" generated from simple precursor systems [6]. Data is often visualized using van Krevelen diagrams to track elemental composition changes (e.g., H:C vs O:C ratios) associated with classic chemical transformations like dehydration and decarboxylation [6].
The application of these techniques in an integrated workflow allows researchers to deconstruct the complexity of NEB, as shown below:
Experimental Protocols and Kinetic Modeling
Establishing a Model System for Maillard Reaction Studies
Controlled model systems are fundamental for isolating variables and studying specific reaction pathways.
- Protocol: Ribose-Amino Acid Model System [6]
- Objective: To investigate the influence of amino acid side chains on Maillard reaction pathways.
- Preparation: Prepare equimolar (e.g., 0.1 M) aqueous solutions of a reducing sugar (e.g., ribose, chosen for its high reactivity) and different amino acids (e.g., glycine, lysine, cysteine, isoleucine) in sealed vials.
- Reaction Conditions: Heat the solutions at a constant temperature (e.g., 100°C) for varying durations (e.g., 2, 4, 6, 10 hours).
- Termination and Analysis: Quench the reactions in an ice bath. Analyze the samples using the techniques described in Section 3.1. FT-ICR-MS analysis of such a system has revealed over 1,400 distinct molecular formulae, demonstrating that reactivity follows the order: lysine > cysteine > isoleucine ≈ glycine [6].
Investigating Ascorbic Acid Browning Kinetics
- Protocol: Ascorbic Acid-Glycine Model System [5]
- Objective: To study the kinetics of NEB between ascorbic acid and glycine under different pH conditions.
- Preparation: Dissolve ascorbic acid (ASA) and glycine (Gly) in buffer solutions adjusted to target pH values (e.g., 4.5, 6.8, 8.0, 9.5).
- Reaction Conditions: Heat the solutions in sealed pressure vials within an oil bath at temperatures ranging from 110°C to 150°C for timed intervals (10-150 minutes).
- Analysis:
- Reactant Depletion: Use RP-HPLC to quantify remaining ASA and Gly [5].
- Product Formation: Measure absorbance at 294 nm (UIPs) and 420 nm (BPs) spectrophotometrically.
Kinetic Modeling and Shelf-Life Prediction
Reaction kinetics are vital for understanding reaction pathways and predicting product shelf-life.
- Kinetic Order: The formation of UIPs (A294) and BPs (A420) in the ASA-Gly system often follows zero-order kinetics, where the rate of formation is constant [5].
- Rate Constant (k): Determined from the slope of the linear regression of absorbance versus time.
- Activation Energy (Ea): Calculated using the Arrhenius equation (k = A e^(-Ea/RT)), which describes the temperature dependence of the reaction rate [7] [5]. Studies on the ASA-Gly system found that an acidic environment (pH 4.5) facilitated UIP formation (lower Ea ~53.76 kJ/mol) but did not strongly promote BP formation (higher Ea ~94.06 kJ/mol) [5].
- Shelf-Life Prediction: The Arrhenius model is widely used to predict the shelf-life of products like milk by extrapolating data from accelerated storage conditions (e.g., higher temperatures) to normal storage temperatures [7].
Table 2: Key Research Reagent Solutions and Their Functions in NEB Studies
| Reagent / Material | Function / Rationale | Example Application |
|---|---|---|
| Reducing Sugars (e.g., Ribose, Glucose, Fructose) | Carbonyl donor in Maillard reaction; highly reactive ribose is often chosen for model studies to accelerate reaction timelines [6]. | Maillard model systems [6] |
| Amino Acids (e.g., Glycine, Lysine, Cysteine) | Amino group donor; side chain structure (e.g., lysine's ε-amino group, cysteine's thiol) dramatically influences pathway and product profile [6] [5]. | Studying amino acid-specific reactivity [6] |
| l-Ascorbic Acid (ASA) | A key reactant in the ascorbic acid oxidation browning pathway; also acts as an antioxidant or pro-oxidant depending on context [5]. | Ascorbic acid browning kinetics [5] |
| Buffer Solutions (e.g., Phosphate) | To control and maintain the pH of the reaction system, a critical parameter influencing reaction pathways and rates [5]. | pH-dependent studies [5] |
| Tea Polyphenols (e.g., Catechins) | Natural antioxidants used to study inhibition of NEB; they trap reactive α-dicarbonyl compounds and Strecker aldehydes [7] [8]. | Inhibiting MRP formation in milk models [7] |
| Isotope-Labelled Compounds (e.g., 13C6-Glucose) | Tracers to elucidate specific reaction pathways and track the incorporation of atoms from precursors into products via techniques like LC-MS [4]. | Metabolic tracing of reaction pathways [4] |
Factors Influencing NEB and Mitigation Strategies
Critical Influencing Parameters
The rate and pathway of NEB reactions are governed by several intrinsic and extrinsic factors:
- pH: This is a master variable. It controls the protonation state of amino groups (nucleophiles) and influences the degradation pathway of ARPs. Acidic conditions generally slow the Maillard reaction but can favor ascorbic acid degradation and specific sugar dehydration pathways [1] [2] [5].
- Temperature and Time: Higher temperatures exponentially accelerate all NEB reactions, as described by the Arrhenius law. The duration of heating or storage directly impacts the extent of browning [2] [7].
- Water Activity (a~w~): NEB rates are typically highest at intermediate water activity (a~w~ ~0.6-0.8), where reactant mobility is sufficient but dilution effects are minimized [2].
- Reactant Type and Concentration: The structure of sugars and amino acids is critical. Pentoses (e.g., ribose) are more reactive than hexoses (e.g., glucose). Among amino acids, lysine (with a second reactive amino group) and cysteine (with a reactive thiol group) are highly reactive [6] [2].
Strategies for Controlling NEB
Based on the understanding of influencing factors, several strategies can be employed to mitigate undesirable browning:
- pH Adjustment: Lowering pH can effectively suppress the Maillard reaction in many systems.
- Temperature Control: Minimizing exposure to high temperatures during processing and storage.
- Use of Inhibitors: Natural polyphenols (e.g., from tea, fruits) are effective inhibitors. They sequester key reactive intermediates, particularly α-dicarbonyl compounds like glyoxal and methylglyoxal, thereby blocking downstream browning pathways [7] [8]. For instance, tea polyphenols have been shown to significantly reduce levels of Strecker aldehydes and HMF in milk model systems [7].
- Packaging Innovations: Antioxidant packaging films (APFs) incorporated with natural polyphenols represent an emerging technology. These films create an active barrier that mitigates oxidation and NEB in food products, contributing to extended shelf-life [9].
The chemistry of non-enzymatic browning, encompassing the Maillard reaction, caramelization, and ascorbic acid oxidation, constitutes a complex yet foundational network of reactions with direct implications for food science, pharmaceutical development, and human health. A deep understanding of the distinct yet sometimes interconnected mechanisms, coupled with robust analytical methodologies and kinetic modeling, empowers researchers to predict and control these processes. Ongoing research continues to elucidate the vast "chemodiversity" of reaction products, their health impacts, and the development of novel, natural strategies for inhibition. This knowledge is crucial for engineering products with superior quality, stability, and safety profiles.
The Maillard reaction, first described by Louis-Camille Maillard in 1912, represents one of the most complex and influential reaction pathways in food chemistry, pharmaceutical research, and human biology [10] [11] [2]. This non-enzymatic browning reaction occurs between nucleophilic amino groups (primarily from amino acids, peptides, or proteins) and carbonyl groups (primarily from reducing sugars) and is responsible for the characteristic flavors, aromas, and colors of thermally processed foods [11]. Beyond its culinary significance, the Maillard reaction has profound implications in human health and disease, contributing to both the formation of beneficial antioxidants and potentially harmful compounds such as advanced glycation end-products (AGEs) [1] [11]. The reaction cascade proceeds through three well-defined stages—initial condensation, intermediate fragmentation, and final polymerization—ultimately yielding melanoidins, the brown, high-molecular-weight nitrogenous polymers that give many processed foods their distinctive appearance [1] [12]. Understanding this complex reaction network is crucial for researchers and drug development professionals seeking to control these processes in food systems, pharmaceutical formulations, and biological environments.
The Three-Stage Maillard Reaction Mechanism
Stage 1: Initial Condensation and Rearrangement
The Maillard reaction cascade begins with the initial condensation stage, characterized by nucleophilic addition and molecular rearrangement. This phase commences when the carbonyl group of a reducing sugar reacts with the free amino group of an amino acid, peptide, or protein to form a reversible N-substituted glycosylamine [10] [11]. This intermediate rapidly undergoes an Amadori rearrangement to form stable 1-amino-1-deoxyketose compounds known as Amadori rearrangement products (ARPs) when aldoses are involved, or Heyns products when ketoses react [1] [11]. Critically, this initial stage is reversible and produces no visible browning, yet establishes the essential foundation for all subsequent reaction pathways [1]. The kinetics and efficiency of this initial condensation are heavily influenced by pH, with alkaline conditions (pH >7) accelerating the reaction by deprotonating amino groups (RNH₃⁺ → RNH₂), thereby increasing their nucleophilicity [10] [11].
Table 1: Key Compounds in the Initial Maillard Reaction Stage
| Compound | Chemical Structure | Formation Pathway | Significance |
|---|---|---|---|
| Glycosylamine | N-substituted glycosylamine | Carbonyl-amine condensation | Initial reversible adduct |
| Amadori Product | 1-amino-1-deoxyketose | Amadori rearrangement | Stable early marker |
| Heyns Product | 2-amino-2-deoxyaldose | Heyns rearrangement | Formed from ketose sugars |
Stage 2: Intermediate Fragmentation and Degradation
The intermediate stage encompasses a complex network of degradation pathways wherein ARPs undergo dehydration, fragmentation, and rearrangement to form highly reactive dicarbonyl compounds [1] [11]. Depending on pH conditions, ARPs follow distinct degradation routes: under acidic or neutral conditions (pH ≤7), they primarily undergo 1,2-enolization, yielding furfurals (from pentoses) or hydroxymethylfurfural (HMF, from hexoses); under alkaline conditions (pH >7), 2,3-enolization dominates, producing reductones and fission products [1]. A pivotal reaction in this stage is Strecker degradation, wherein α-dicarbonyl compounds react with amino acids to form Strecker aldehydes (with one less carbon atom) and aminoketones, generating important aroma compounds while simultaneously degrading the original amino acid [1] [11]. This stage also produces a diverse array of flavor-contributing volatiles including pyrazines (toasted notes), furans (caramel-like), and thiophenes (meaty aromas) [1]. The intermediate stage marks the beginning of visible browning, with chromophores absorbing at 294 nm indicating its progression [6].
Diagram 1: Intermediate stage fragmentation pathways
Stage 3: Final Polymerization to Melanoidins
The final stage of the Maillard reaction involves aldol condensation and heterocyclic polymerization of reactive intermediates from the second stage, leading to the formation of high-molecular-weight, brown-colored nitrogenous polymers known as melanoidins [1] [12]. These complex macromolecules, characterized by heterocyclic nitrogenous structures, are responsible for the characteristic brown color in thermally processed foods and exhibit molecular weights ranging from several thousand to over 100,000 Daltons [12]. Melanoidins form through aldehyde-amine condensation between various reactive intermediates generated during the second stage, particularly dicarbonyl compounds and Strecker degradation products [12]. The formation of these brown pigments is typically monitored by measuring absorbance at 420 nm, which specifically indicates advanced browning associated with the final stage [6]. Beyond their color contribution, melanoidins exhibit diverse biological activities, including antioxidant, antimicrobial, and prebiotic properties, though their environmental persistence can pose challenges when released as industrial waste products [12].
Experimental Analysis and Monitoring Techniques
Analytical Approaches for Maillard Reaction Monitoring
Advanced analytical techniques are essential for monitoring the complex Maillard reaction cascade and characterizing its diverse products. The choice of methodology depends on the specific reaction stage and compounds of interest, with each technique offering distinct advantages for particular applications.
Table 2: Analytical Methods for Monitoring Maillard Reaction Progression
| Technique | Application | Target Compounds | Sensitivity |
|---|---|---|---|
| Spectrophotometry (294 nm) | Intermediate stage monitoring | Chromophores | Moderate |
| Spectrophotometry (420 nm) | Final stage browning | Melanoidins | Moderate |
| HPLC-MS/LC-MS | Quantitative analysis | HMF, furosine, specific MRPs | High |
| GC-MS | Volatile compound profiling | Strecker aldehydes, furans, pyrazines | High |
| FTIR | Structural characterization | Functional group changes | Moderate |
| FT-ICR-MS | Non-targeted analysis | Molecular formulae of MRPs | Very High |
| ELISA | Specific AGE detection | CML, other AGEs | Variable |
| NMR | Structural elucidation | Molecular structure | High |
Research Reagent Solutions Toolkit
Table 3: Essential Research Reagents for Maillard Reaction Studies
| Reagent Category | Specific Examples | Research Application | Function |
|---|---|---|---|
| Reducing Sugars | D-glucose, D-ribose, fructose | Model system studies | Carbonyl donor |
| Amino Acids | Glycine, lysine, cysteine | Reactivity studies | Amino group donor |
| Buffers | Phosphate, carbonate buffers | pH control studies | Reaction condition control |
| Metal Salts | Cu²⁺, Fe²⁺, Zn²⁺ ions | Catalysis studies | Reaction catalysts |
| Antioxidants | Ascorbic acid | Inhibition studies | Free radical scavengers |
| Inhibitors | Sulfur dioxide | Control studies | Carbonyl group blocker |
Factors Influencing the Maillard Reaction Cascade
The progression and outcome of the Maillard reaction are profoundly influenced by multiple physical and chemical parameters that researchers must carefully control in experimental settings. Understanding these factors is essential for designing reproducible studies and manipulating reaction pathways toward desired outcomes.
Temperature and Time: The Maillard reaction typically proceeds rapidly between 140-165°C (280-330°F), with higher temperatures accelerating all stages of the reaction cascade [10]. Reaction time similarly influences the extent of browning, with longer durations favoring the formation of advanced MRPs and melanoidins [11] [13].
pH Conditions: pH significantly impacts reaction kinetics and pathway selection by influencing the protonation state of amino groups [11]. Alkaline conditions (pH >7) dramatically increase reaction rates by deprotonating amino groups, enhancing their nucleophilicity, and favoring different degradation pathways from ARPs [1] [11].
Water Activity (aᵥ): The Maillard reaction exhibits maximum browning rates at intermediate water activities (aᵥ = 0.3-0.7), where sufficient molecular mobility exists without excessive dilution of reactants [12]. Low-moisture systems generally exhibit enhanced browning due to concentrated reactants.
Reactant Characteristics: The specific chemical properties of reactants profoundly influence reaction pathways and products. Research using FT-ICR-MS analysis has demonstrated that amino acid reactivity follows the order: lysine > cysteine > isoleucine ≈ glycine [6]. Similarly, reducing sugars vary in reactivity, with pentoses (e.g., ribose) generally being more reactive than hexoses (e.g., glucose) [6].
Metal Ions: Transition metal ions including copper (Cu²⁺), iron (Fe²⁺/Fe³⁺), and zinc (Zn²⁺) can catalyze Maillard reactions by facilitating electron transfer processes and accelerating the oxidation of ARPs [11]. Additionally, melanoidins can complex with metal ions due to their anionic character, influencing both reaction progression and environmental behavior [12].
Diagram 2: Key factors influencing Maillard reaction kinetics
Implications and Applications
The Maillard reaction cascade extends far beyond food chemistry, with significant implications across pharmaceutical, medical, and environmental domains. In food systems, the reaction creates desirable flavors and colors but can also reduce nutritional value by decreasing protein quality and essential amino acid availability [1] [11]. The dual nature of MRPs presents both opportunities and challenges—melanoidins exhibit beneficial antioxidant properties at low concentrations but can form potentially harmful compounds including acrylamide (a probable carcinogen), heterocyclic amines, and advanced glycation end-products (AGEs) [10] [11] [13]. In biological systems, Maillard-like reactions contribute to protein aging and have been implicated in diabetes and other age-related diseases through the formation of AGEs that activate inflammatory and oxidative stress responses [11] [13]. Environmental concerns arise from industrial melanoidins in distillery wastewater, where these recalcitrant compounds can complex with heavy metals and other pollutants, posing ecological risks through aquatic toxicity and inhibition of microbial activity [12]. Current research focuses on controlling specific Maillard pathways to enhance beneficial aspects while minimizing formation of detrimental compounds, employing strategies such as reactant modification, process condition optimization, and the use of specific inhibitors like asparaginase to reduce acrylamide formation [10] [13].
This technical guide delineates the foundational chemical mechanisms initiating the Maillard reaction, a non-enzymatic browning process of significant import to food science, therapeutics, and drug development. The cascade commences with the nucleophilic addition of an amine to a carbonyl, progressing through metastable Schiff base formation, and culminating in the Amadori rearrangement to establish stable ketoamine derivatives. Within biological systems, these reactions underpin the formation of advanced glycation end-products (AGEs), which are implicated in the pathophysiology of diabetes and aging. This whitepaper provides an in-depth mechanistic analysis, summarizes critical quantitative parameters, details standardized experimental protocols, and visualizes the core reaction pathways to serve researchers and scientists in the field.
The Maillard reaction, first described by Louis-Camille Maillard in 1912, represents a complex network of chemical interactions between reducing sugars and nucleophilic amine groups primarily from amino acids, peptides, or proteins [2] [14]. For researchers in drug development, this reaction is a double-edged sword. It is crucial for understanding protein glycation in vivo, a process linked to the molecular aging of proteins and the pathogenesis of chronic diseases such as diabetes, where subsequent AGEs contribute to cellular dysfunction [15] [14]. The initial stages—nucleophilic addition, Schiff base formation, and the Amadori rearrangement—are critical control points that determine the trajectory of subsequent reaction pathways and the nature of the final products [2]. A meticulous understanding of these steps is therefore paramount for designing interventions to inhibit deleterious glycation or for harnessing the reaction for therapeutic conjugate synthesis.
Mechanistic Analysis of Key Initial Steps
Nucleophilic Addition: The Initial Trigger
The Maillard reaction is initiated by a fundamental step in carbonyl chemistry: nucleophilic addition [16] [2].
- Electronic Drivers: The carbonyl group (C=O) is highly polarized due to the significant electronegativity difference between oxygen (3.5) and carbon (2.5). This results in a partial positive charge (δ+) on the carbon atom, rendering it electrophilic, and a partial negative charge (δ-) on the oxygen atom [16] [17]. This electron-deficient carbon is susceptible to attack by nucleophiles.
- The Mechanism: The lone pair of electrons on the nitrogen atom of a primary amino group (R-NH₂) acts as the nucleophile, attacking the electrophilic carbonyl carbon. This leads to the formation of a reversible, tetrahedral carbinolamine intermediate (also known as a hemiaminal), as the carbon's hybridization changes from sp² (trigonal planar) to sp³ (tetrahedral) [16] [14].
- Influence of Reaction Medium: The rate of this nucleophilic addition is highly dependent on pH. A higher pH favors the deprotonated, more nucleophilic form of the amine (R-NH₂) over its protonated conjugate acid (R-NH₃⁺), thereby accelerating the reaction [2].
Schiff Base Formation: The First Condensate
The carbinolamine intermediate is unstable and readily undergoes acid- or base-catalyzed dehydration.
- Dehydration: This step involves the loss of a water molecule, leading to the formation of an N-substituted glycosylamine, more commonly referred to as a Schiff base (R₁R₂C=NR₃) [18] [14]. The formation of this C=N bond is a condensation reaction.
- Reversibility: The Schiff base formation is a reversible process. The dehydration step is often the rate-limiting step in imine formation, and the equilibrium can be driven forward by conditions that remove water, such as heating [14].
Amadori Rearrangement: Toward Stability
The Schiff base derived from an aldose sugar undergoes a critical intramolecular rearrangement known as the Amadori rearrangement.
- The Rearrangement Process: This acid- or base-catalyzed step involves the irreversible migration of the carbonyl group from the C1 position of the original sugar to the C2 position. This transforms the relatively unstable aldosylamine (Schiff base derived from an aldose) into a more stable 1-amino-1-deoxy-2-ketose, known as the Amadori product [14].
- Significance: The formation of the Amadori product is a pivotal commitment to the Maillard reaction pathway. While more stable than the Schiff base, the Amadori product serves as a key precursor for a multitude of subsequent degradation and condensation reactions. Its decomposition under physiological or heating conditions leads to the formation of highly reactive α-dicarbonyl compounds (e.g., 3-deoxyglucosone) and other intermediates that ultimately yield flavor compounds, aromas, and both beneficial (antioxidants) and deleterious (AGEs) products [15] [2].
The following diagram illustrates the logical sequence and chemical structures involved in this three-stage process.
Quantitative Data and Kinetic Parameters
Understanding the kinetics and thermodynamics of these initial steps is crucial for controlling the Maillard reaction in experimental and industrial contexts.
Table 1: Factors Influencing the Rate of Nucleophilic Addition to Carbonyls
| Factor | Effect on Reaction Rate | Underlying Principle & Research Context |
|---|---|---|
| Carbonyl Reactivity | Aldehydes > Ketones | Steric hindrance: Aldehydes have one R group vs. two in ketones. Electronic effects: Alkyl groups in ketones are slightly electron-donating, reducing the carbonyl carbon's electrophilicity [16]. |
| Electronic Effects | Electron-withdrawing groups (e.g., -CF₃) increase rate. Electron-donating groups decrease rate. | Adjacent electron-withdrawing groups increase the partial positive charge on the carbonyl carbon, enhancing its electrophilicity. This principle explains the high reactivity of trichloroacetaldehyde [16]. |
| pH | Rate increases with higher pH. | A higher pH increases the concentration of the deprotonated, more nucleophilic amine species (R-NH₂) relative to its protonated form (R-NH₃⁺) [2]. |
| Nucleophile Basicity | Stronger nucleophiles (e.g., H⁻, R⁻) give irreversible addition. Weaker nucleophiles (e.g., HO⁻, ROH) give reversible addition. | The position of the equilibrium is determined by the relative basicity of the nucleophile versus the alkoxide in the tetrahedral intermediate. Irreversible addition with strong bases (e.g., Grignard reagents) is favored by >20 pKa units [16]. |
Table 2: Experimental Data on Amadori Compound Decomposition
| Parameter | Experimental Condition | Observed Effect / Quantitative Outcome | Research Context |
|---|---|---|---|
| Buffer System | 0.2 M Phosphate vs. Hepes buffer, pH 7.4, 37°C | Shorter half-life of model Amadori compound (Nα-formyl-Nε-fructoselysine) in phosphate buffer [15]. | Phosphate anions act as general acid-base catalysts, accelerating the decomposition of Amadori products to reactive intermediates like 3-deoxyglucosone [15]. |
| Oxygen Presence | Aerobic vs. Anaerobic conditions, 90°C, pH 7.0 | Accelerated decomposition and sixfold increase in Maillard browning rate under aerobic conditions [19]. | Oxygen acts as a catalyst and participant in oxidative degradation pathways, propagating the reaction and leading to increased formation of advanced products [19]. |
| Sugar Type | Culture medium with Tryptone, 90°C | Severe growth inhibition of A. pernix with reducing sugars (glucose, fructose); No inhibition with non-reducing sugars (sucrose, trehalose) [19]. | Confirms that a free carbonyl group is essential for the initial nucleophilic addition step. Non-reducing sugars cannot form Schiff bases and Amadori products under these conditions. |
Detailed Experimental Protocols
Protocol: Monitoring Schiff Base and Amadori Product Formation In Vitro
This protocol is adapted from methodologies used to study the early Maillard reaction in model systems [15] [14].
Objective: To synthesize and characterize the formation of Schiff base and Amadori products from a model sugar and amino acid.
The Scientist's Toolkit: Essential Reagents and Materials
| Reagent / Material | Function / Rationale |
|---|---|
| D-Glucose (or other reducing sugar) | Provides the electrophilic carbonyl reactant. |
| L-Lysine (or a simple primary amine like glycine) | Provides the nucleophilic amino group. |
| Phosphate Buffer (0.1-0.5 M, pH 7.0-8.0) | Maintains physiological pH; phosphate ions can catalyze rearrangement and decomposition. |
| Hepes Buffer (0.1-0.5 M, pH 7.4) | An alternative, non-catalytic buffer for studying specific buffer effects. |
| RP-HPLC System with UV/Vis and MS Detectors | For separating and identifying reaction intermediates and products. Amadori products can be detected by UV absorption. |
| FTIR Spectrometer | To monitor the appearance of the C=N stretch (~1640 cm⁻¹) for the Schiff base. |
| Heating Block or Incubator | To maintain precise temperature (e.g., 37°C for physiological studies or 50-90°C for accelerated models). |
Methodology:
- Solution Preparation: Prepare 100 mM solutions of D-glucose and L-lysine in both 0.2 M phosphate buffer (pH 7.4) and 0.2 M Hepes buffer (pH 7.4).
- Reaction Initiation: Mix equal volumes of the sugar and amino acid solutions in sealed vials. Include controls containing only sugar or only amino acid in buffer.
- Incubation: Incubate the reaction mixtures at a defined temperature (e.g., 37°C or 60°C) for varying time periods (e.g., 0, 1, 3, 7 days).
- Sample Analysis:
- Schiff Base Monitoring (FTIR): At each time point, take an aliquot, lyophilize, and analyze by FTIR. Track the emergence and intensity of the imine (C=N) absorption band.
- Amadori Product Quantification (HPLC): Directly inject filtered aliquots onto an RP-HPLC column (e.g., C18). Use a water-acetonitrile gradient and monitor at 220-280 nm. Compare retention times and mass spectra with synthesized standards if available.
- Kinetic Analysis: Plot the concentration of the Amadori product (from HPLC peak area) versus time to determine the rate of formation under different buffer and temperature conditions.
Protocol: Assessing the Biological Impact of Maillard Reaction Products (MRPs)
This protocol is based on studies investigating the cytotoxic effects of pre-formed MRPs on microbial growth [19].
Objective: To evaluate the growth-inhibitory effect of MRPs generated in a culture medium.
Methodology:
- MRP Generation: Prepare a base medium containing tryptone (4.0 g/L) and a specific carbohydrate (e.g., 3.0 g/L D-glucose). Heat the medium at 90°C for a defined period (e.g., 2-4 hours) to generate MRPs. A control medium with tryptone alone should be heated similarly.
- Browning Measurement: After heating, cool the medium and measure the absorbance at 420 nm (OD₄₂₀) to quantify the level of advanced Maillard browning products [19].
- Culture Inoculation: Inoculate the pre-heated media with a standardized inoculum of a sensitive organism, such as the hyperthermophile Aeropyrum pernix.
- Growth Monitoring: Incubate the culture at the organism's optimal growth temperature (90°C for A. pernix) under aerobic conditions. Monitor cell growth over time by measuring optical density at 660 nm (OD₆₆₀).
- Data Interpretation: Compare the maximum cell density (ΔOD₆₆₀) and growth rate in the glucose-supplemented MRP-rich medium versus the tryptone-only control. A significant reduction in growth correlates with the toxicity of the generated MRPs.
The workflow for this bioassay is outlined below.
Research Implications and Future Directions
The precise characterization of the Maillard reaction's initial steps provides a foundational framework for several research and development avenues.
In drug development and therapeutic science, understanding these mechanisms is critical for mitigating protein glycation in vivo, a key contributor to diabetic complications and age-related diseases [15] [14]. Research focuses on designing nucleophilic traps (e.g., pyridoxamine) that scavenge reactive dicarbonyl intermediates derived from Amadori product decomposition, thereby preventing the formation of pathogenic AGEs [20].
Conversely, the antioxidant properties of certain MRPs, particularly melanoidins, are being explored for stabilizing pharmaceuticals and creating functional foods. These high molecular weight compounds can donate hydrogen atoms, chelate pro-oxidant metal ions, and break radical chains, thereby protecting against oxidative degradation [20].
Future research will continue to refine kinetic models of these pathways, identify specific AGE structures linked to disease states, and develop highly selective inhibitors of the most deleterious glycation pathways. The experimental tools and mechanistic insights outlined in this whitepaper are essential for driving these innovations forward.
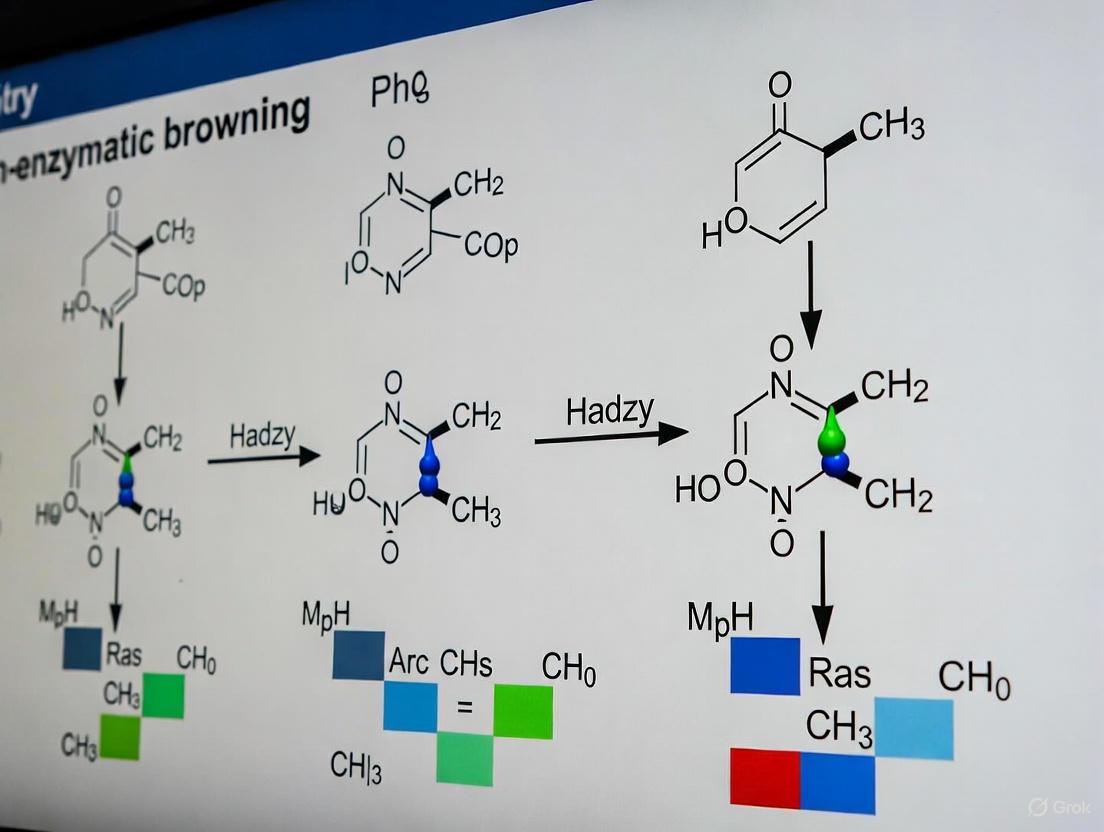
The Intermediate Stage of the Maillard reaction represents a critical branching point where initial condensation products evolve into the compounds that define the sensory and safety properties of processed foods. This phase is mechanistically dominated by the formation of highly reactive α-dicarbonyl compounds (α-DCs), which serve as essential intermediates in parallel pathways leading to both desirable aromas and potentially hazardous substances [11]. The Strecker degradation, a pivotal reaction within this stage, converts amino acids into characteristic aroma-active aldehydes while simultaneously providing reactants that facilitate the formation of nitrogen-containing heterocycles and other flavor compounds [21]. Concurrently, these same α-dicarbonyl intermediates can react with specific amino acids like asparagine to form acrylamide, a processing contaminant of significant health concern [22]. Understanding the kinetic and thermodynamic factors that govern these competing pathways is essential for researchers aiming to control Maillard reaction outcomes in food and pharmaceutical formulations. This review synthesizes current knowledge on the mechanisms, key intermediates, and experimental approaches relevant to this complex reaction network, providing a scientific foundation for targeted intervention strategies in product development.
The Mechanism of Strecker Degradation and Aroma Formation
The Strecker degradation is a cornerstone reaction of the intermediate Maillard stage, responsible for converting amino acids into volatile aldehydes that contribute significantly to food aromas. The mechanism involves the reaction between an α-dicarbonyl compound (e.g., glyoxal, methylglyoxal, diacetyl) and an α-amino acid [21]. This process results in deamination and decarboxylation of the amino acid, producing an aldehyde containing one fewer carbon atom than the original amino acid, along with an α-aminocarbonyl compound [11].
Key Reactive Intermediates: α-Dicarbonyl Compounds
α-Dicarbonyl compounds (α-DCs) such as glyoxal (GO), methylglyoxal (MGO), and diacetyl (DA) are formed primarily through sugar fragmentation during the non-enzymatic browning reactions [23]. These compounds are yellow-colored, low molecular weight organic compounds containing two carbonyl groups on adjacent carbon atoms. Their high electrophilicity makes them exceptionally reactive toward nucleophilic amino acid side chains, enabling them to participate in multiple Maillard pathways simultaneously [24]. In model systems, the concentrations of these key intermediates vary significantly based on reactant composition, with reported ranges of 5.92 to 39.10 μg/mL for GO, 3.66 to 151.88 μg/mL for MGO, and 1.10 to 6.12 μg/mL for DA under controlled conditions [23].
Strecker Aldehyde Formation and Sensory Impact
The aldehydes produced via Strecker degradation contribute distinct sensory notes depending on their precursor amino acids. For instance, methional (from methionine) imparts a cooked potato aroma, phenylacetaldehyde (from phenylalanine) provides honey-like notes, and 3-methylbutanal (from leucine) contributes malty, chocolate aromas [25]. The specific aroma profile generated is thus directly determined by the amino acid composition of the system [25].
Table 1: Characteristic Aromas from Strecker Degradation of Selected Amino Acids
| Amino Acid Precursor | Strecker Aldehyde | Characteristic Aroma |
|---|---|---|
| Valine | 2-Methylpropanal | Rye bread, pungent chocolate |
| Leucine | 3-Methylbutanal | Sweet chocolate, cooked cheese |
| Isoleucine | 2-Methylbutanal | Musty, cooked cheese |
| Phenylalanine | Phenylacetaldehyde | Violet, lilac, honey-like |
| Methionine | Methional | Potato-like |
Environmental factors significantly influence Strecker aldehyde accumulation. Studies in wine matrices have demonstrated that pH exerts a particularly strong effect, with higher pH values (e.g., 3.8 vs. 3.4) promoting greater accumulation of phenylacetaldehyde and methional during oxidation [26]. Additionally, the presence of polyphenols and sulfur dioxide can limit free aldehyde concentrations through adduct formation, effectively modulating the sensory impact of these compounds [26].
α-Dicarbonyls as a Link Between Aromas and Hazardous Compounds
The central role of α-dicarbonyl compounds creates a mechanistic link between the formation of desirable aromas and potentially hazardous substances. These reactive intermediates participate in multiple parallel pathways, acting as a control point that influences both sensory quality and product safety [24].
Co-Formation of Aroma Compounds and Advanced Glycation End Products (AGEs)
Recent research has quantitatively demonstrated that α-dicarbonyl intermediates mediate the co-formation of aroma compounds and advanced glycation end products (AGEs). In glucose-lysine model systems heated to 150°C, the critical period for concurrent formation of both compound classes occurs between 20 to 30 minutes of heating [24]. During this window, concentrations of both pyrazines (aroma compounds) and AGEs (specifically Nε-carboxyethyllysine, CEL) increase significantly, indicating shared precursor pathways and competitive reaction kinetics.
The dynamic interconversion between these pathways is governed by the availability of key α-dicarbonyls. Glyoxal (GO) and methylglyoxal (MGO) participate in Strecker degradation, promoting pyrazine synthesis, while simultaneously reacting with lysine residues to form AGEs through cross-linking reactions [24]. This mechanistic overlap presents a significant challenge for product formulation, as interventions targeting hazardous compound reduction may inadvertently impact flavor development.
Acrylamide Formation Pathway
A particularly concerning pathway involving α-dicarbonyl compounds leads to the formation of acrylamide. This process occurs when the amino acid asparagine reacts with reducing sugars or α-dicarbonyl intermediates at temperatures typically above 120°C [22]. The reaction proceeds through the Maillard pathway, with asparagine initially condensing with carbonyl compounds to form Schiff bases, which then undergo decarboxylation and subsequent reactions to yield acrylamide [11].
The formation of acrylamide is particularly favored in carbohydrate-rich foods processed at high temperatures, such as potato products, cereals, and coffee [27]. Its classification as a potential human carcinogen by international health organizations has driven extensive research into mitigation strategies [22].
Table 2: Key α-Dicarbonyl Compounds and Their Roles in Maillard Reaction Pathways
| α-Dicarbonyl Compound | Formation Pathway | Role in Aroma Formation | Role in Hazardous Compound Formation |
|---|---|---|---|
| Glyoxal (GO) | Sugar fragmentation, lipid oxidation | Strecker degradation of various amino acids | Formation of CML, protein cross-linking |
| Methylglyoxal (MGO) | Glucose degradation, Maillard intermediates | Strecker degradation of specific amino acids | Formation of CEL, major precursor to AGEs |
| Diacetyl (DA) | Sugar degradation, microbial activity | Contributes to buttery aromas | Potential precursor to other reactive species |
Experimental Approaches and Analytical Methodologies
Research into the intermediate Maillard stage requires sophisticated analytical approaches to track multiple reaction products simultaneously and elucidate complex reaction mechanisms.
Model System Design
Well-controlled model systems provide the foundation for studying Maillard reaction pathways. Typical approaches involve preparing equimolar solutions (e.g., 0.1 M) of reducing sugars and amino acids in appropriate buffers, with reactions conducted at elevated temperatures (e.g., 160°C) for defined periods (e.g., 2 hours) [23]. The pH is carefully controlled, as it significantly influences reaction kinetics and product distribution, with many studies conducted at pH 9 to accelerate the Maillard reaction for analytical purposes [23].
Analysis of α-Dicarbonyl Compounds
Quantification of α-dicarbonyl compounds typically involves derivatization with o-phenylenediamine (OPD) to form stable quinoxaline derivatives, which can be extracted with organic solvents (e.g., ethyl acetate) and analyzed by gas chromatography with nitrogen phosphorous detection (GC-NPD) or mass spectrometry [23]. This approach allows for sensitive detection of these key intermediates at concentrations as low as 0.5 μg/mL in model systems [23].
Analysis of Volatile Aroma Compounds
Volatile aroma compounds are commonly analyzed using headspace solid-phase microextraction (HS-SPME) followed by gas chromatography-mass spectrometry (GC-MS) [23]. This technique enables the identification and quantification of a wide range of volatile compounds, including Strecker aldehydes, pyrazines, furans, and other heterocyclic compounds. Researchers often use internal standards such as methyl cinnamate for quantification and alkane standards for retention index calculation [23].
Tracing Reaction Pathways with Isotopic Labeling
Advanced mechanistic studies employ isotopically labeled compounds to trace the fate of specific atoms during the Maillard reaction. The Carbon Module Labeling (CAMOLA) technique, using uniformly 13C-labeled glucose, provides insights into the fragmentation and recombination of sugar-derived carbon atoms during the formation of both aroma compounds and hazardous products [24]. This approach has revealed that approximately 47-49% of the carbon in certain pyrazines originates from the amino acid rather than the sugar precursor [24].
The Researcher's Toolkit: Essential Reagents and Methodologies
Table 3: Key Research Reagents and Analytical Tools for Investigating the Intermediate Maillard Stage
| Reagent / Material | Function / Application | Representative Examples |
|---|---|---|
| α-Dicarbonyl Standards | Quantification and method validation | Glyoxal, Methylglyoxal, Diacetyl [23] |
| Strecker Amino Acids | Study of specific degradation pathways | Valine, Leucine, Isoleucine, Phenylalanine, Methionine [26] [25] |
| 13C-labeled Sugars | Isotopic tracing of reaction pathways | D-glucose (U13C6) for CAMOLA technique [24] |
| Derivatization Reagents | Stabilization and detection of carbonyl compounds | o-Phenylenediamine (OPD) for α-dicarbonyl analysis [23] |
| SPME Fibers | Extraction of volatile compounds for GC-MS | DVB/CAR/PDMS (50 μm) for aroma compound extraction [23] |
| AGEs Standards | Quantification of advanced glycation endproducts | Nε-carboxymethyllysine (CML), Nε-carboxyethyllysine (CEL) [24] |
Reaction Pathway Visualization
The intermediate stage of the Maillard reaction, governed by Strecker degradation and α-dicarbonyl chemistry, represents a critical control point for researchers aiming to balance sensory quality with product safety. The demonstrated co-formation of aroma compounds and hazardous products through shared reactive intermediates presents both a challenge and an opportunity for scientific intervention. Future research directions should focus on precision control of Maillard pathways through tailored reactant compositions, optimized processing parameters, and potentially the application of selective inhibitors that can decouple desirable flavor formation from the generation of toxic compounds. The continued development of advanced analytical techniques, particularly those enabling real-time monitoring of multiple reaction products simultaneously, will be essential for building predictive models that can guide product formulation and process optimization across food and pharmaceutical applications.
The Maillard reaction, a form of non-enzymatic browning, represents one of the most complex reaction networks in food chemistry, fundamentally transforming the sensory, nutritional, and functional properties of processed foods [11] [28]. This reaction proceeds through three distinct stages: the initial formation of glycosylamines and Amadori rearrangement products, an intermediate stage yielding numerous flavor-active and carbonyl compounds, and a final polymerization stage that produces brown pigments known as melanoidins [1] [11]. These high-molecular-weight nitrogenous polymers are not only responsible for the desirable brown color in baked goods, roasted coffee, and grilled meats but also exhibit significant biological activities with implications for human health and nutrition [1] [29]. Despite their fundamental importance, melanoidins remain among the least characterized Maillard reaction products (MRPs) due to their structural heterogeneity and macromolecular nature [1]. This whitepaper provides an in-depth technical examination of the polymerization processes, structural characteristics, analytical methodologies, and research protocols essential for investigating these complex brown pigments within the broader context of non-enzymatic browning research.
Chemical Mechanisms of Polymerization
The formation of melanoidins represents the culmination of the Maillard reaction, where reactive intermediates generated in earlier stages undergo complex polymerization reactions. This process involves multiple chemical pathways that collectively transform low-molecular-weight intermediates into heterogeneous macromolecular structures.
Precursors and Reaction Pathways
The final stage of the Maillard reaction is initiated by the diverse pool of reactive intermediates generated during the intermediate stage, including furans, pyrroles, pyrazines, carbonyls, and other heterocyclic compounds [1] [11]. These compounds undergo condensation and polymerization through aldol condensation, carbonyl-amine polymerization, and free radical reactions [11]. The specific polymerization pathways are highly dependent on reaction conditions, particularly pH, which directs the enolization route of Amadori products [1]. Under alkaline conditions (pH >7), Amadori products primarily undergo 2,3-enolization, producing reductones and fission products that readily polymerize [1]. In contrast, acidic conditions (pH ≤7) favor 1,2-enolization, leading to the formation of furfurals (from pentoses) or hydroxymethylfurfural (HMF, from hexoses) [1] [30]. These furanic compounds subsequently participate in polymerization reactions with amino compounds, ultimately forming melanoidins [1].
Table 1: Key Intermediate Compounds in Melanoidin Formation
| Intermediate Type | Specific Compounds | Formation Pathway | Role in Polymerization |
|---|---|---|---|
| Furanic Compounds | Furfural, Hydroxymethylfurfural (HMF) | 1,2-enolization of Amadori products under acidic conditions | Act as electrophiles in polymerization with amino compounds |
| α-Dicarbonyl Compounds | Diacetyl, Pyruvaldehyde | Sugar fragmentation and degradation | Highly reactive intermediates that cross-link with amines |
| Strecker Aldehydes | Aldehydes from amino acid degradation | Strecker degradation of amino acids with dicarbonyls | Contribute to flavor and participate in condensation |
| Heterocyclic Compounds | Pyrroles, Pyrazines | Cyclization and rearrangement reactions | Provide nitrogen atoms and contribute to chromophore formation |
Structural Characteristics of Melanoidins
Melanoidins are characterized as brown nitrogenous polymers with complex and poorly defined structures [10]. Current research suggests they consist of skeleton structures formed from sugar fragments that incorporate nitrogen from amino acids, with proteinaceous materials possibly integrated into the polymer framework [29]. These macromolecules typically contain chromophoric systems based on conjugated double bonds and unsaturated carbonyl structures that are responsible for their characteristic brown color [29]. The structural diversity of melanoidins is immense, varying significantly based on the specific sugar-amino acid precursor system and the reaction conditions employed during their formation [6]. This heterogeneity presents substantial analytical challenges for structural elucidation.
Figure 1: Reaction Pathway from Early Maillard Intermediates to Melanoidin Formation
Analytical Methodologies for Melanoidin Characterization
The complex and heterogeneous nature of melanoidins necessitates the application of multiple complementary analytical techniques to characterize their structural properties, molecular weight distribution, and functional characteristics.
Spectroscopic and Colorimetric Methods
Spectrophotometric analysis remains a fundamental approach for monitoring melanoidin formation, typically measuring absorbance at 294 nm for intermediate products and 420 nm for advanced browning [1] [6]. For quantitative assessment, Martins and van Boekel established an extinction coefficient of 1.0 L/(mol·cm) for melanoidins in glucose/glycine model systems, which has been widely adopted for relative comparisons across different reaction systems [1]. Colorimetric measurements in the CIE Lab* color space provide additional information on visual browning and have been applied to various food products, including spaghetti, crayfish tails, and apple juice [1]. Fluorescence spectroscopy can distinguish between different browning stages, as demonstrated in thermally treated apple juice, though it only detects fluorescent MRPs and provides no information on non-fluorescent species or specific cross-link structures [1].
Advanced Structural Analysis Techniques
For more detailed structural characterization, high-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS) are employed to detect and quantify specific Maillard reaction intermediates and products, including HMF, furosine, and amino nitrogen compounds [1]. Gas chromatography-mass spectrometry (GC-MS) is particularly valuable for identifying low-molecular-weight volatile compounds that may participate in polymerization reactions, such as Strecker degradation products [1]. In recent years, ultrahigh-resolution Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) has emerged as a powerful tool for the compositional characterization of complex MRPs, enabling the assignment of unambiguous molecular formulae to hundreds of distinct reaction products in Maillard model systems [6]. This technique has revealed more than 1,493 distinct molecular formulae across different amino acid-ribose model systems, demonstrating the remarkable chemodiversity of MRPs [6].
Table 2: Analytical Methods for Melanoidin Characterization
| Method Category | Specific Techniques | Key Applications | Limitations |
|---|---|---|---|
| Spectrophotometric | UV-Vis at 294 nm & 420 nm | Browning intensity measurement, reaction kinetics | Non-specific, relative comparisons only |
| Colorimetric | CIE Lab* color space | Visual browning correlation in food products | Empirical, equipment-dependent |
| Separation Science | HPLC, LC-MS | Quantification of specific MRPs (HMF, furosine) | May miss non-extractable or HMW fractions |
| Volatile Analysis | GC-MS | Identification of low-MW flavor compounds & precursors | Requires volatility or derivatization |
| Advanced MS | FT-ICR-MS | Molecular formula assignment, chemodiversity assessment | Limited structural information, complex data analysis |
| Structural Probes | Fluorescence spectroscopy | Detection of fluorescent MRPs, reaction staging | Insensitive to non-fluorescent compounds |
Experimental Protocols for Melanoidin Research
Well-designed experimental protocols are essential for reproducible research on melanoidin formation and characterization. The following section outlines standardized methodologies for establishing Maillard model systems and monitoring melanoidin development.
Establishing Maillard Model Systems
Amino acid-sugar model systems provide a controlled environment for studying melanoidin formation. A typical protocol involves preparing equimolar (0.1 M) solutions of a selected reducing sugar (e.g., glucose, ribose) and amino acid (e.g., glycine, lysine) in appropriate buffers to control pH [6]. The choice of amino acid significantly influences the reaction pathway and products, with lysine and cysteine demonstrating particularly high reactivity due to their nucleophilic side chains [6]. The reaction mixture is heated at controlled temperatures (typically 100°C) for varying durations (2-10 hours) to track reaction progression [6]. For more complex systems, protein-polysaccharide models can be employed using hydrolyzed proteins (e.g., whey protein, squid skin) combined with polysaccharides (e.g., chitosan) to better simulate food matrices [29].
Monitoring Melanoidin Formation
The browning kinetics can be monitored by measuring absorbance at 294 nm (intermediate MRPs) and 420 nm (advanced browning and melanoidins) at regular intervals during heating [1] [6]. For quantitative assessment of melanoidin concentration, the extinction coefficient method (ε = 1.0 L/[mol·cm] at 420 nm) can be applied to glucose/glycine systems, though this requires validation for different precursor systems [1]. FT-ICR-MS analysis provides comprehensive molecular-level information on the reaction products, with samples typically diluted in methanol or acetonitrile prior to direct infusion [6]. Data processing involves formula assignment using compositional networks and isotopic fine structure validation to eliminate false assignments [6].
Figure 2: Experimental Workflow for Melanoidin Formation Studies
Research Reagent Solutions and Essential Materials
The investigation of melanoidin formation requires specific chemical reagents, analytical standards, and specialized equipment to ensure reproducible and interpretable results.
Table 3: Essential Research Reagents and Materials for Melanoidin Studies
| Category | Specific Items | Function/Application | Technical Notes |
|---|---|---|---|
| Sugar Precursors | D-Glucose, D-Ribose, Fructose, Lactose | Carbonyl group donors in Maillard reaction | Ribose demonstrates high reactivity; purity >99% recommended |
| Amino Acid Precursors | Glycine, L-Lysine, L-Cysteine, L-Arginine | Amino group donors in Maillard reaction | Lysine and cysteine show enhanced reactivity due to side chains |
| Buffers & pH Control | Phosphate buffers (various pH) | Reaction environment control | Critical for directing enolization pathway (1,2- vs 2,3-enolization) |
| Analytical Standards | HMF, Furosine, N-ε-carboxymethyllysine (CML) | Quantification of specific MRPs | HPLC or LC-MS calibration and method validation |
| Chromatography | HPLC/LC-MS systems, C18 columns | Separation and quantification of MRPs | Enable specific MRP analysis beyond overall browning |
| Mass Spectrometry | FT-ICR-MS or high-resolution MS | Molecular formula assignment | Essential for comprehensive MRP profiling |
| Spectroscopy | UV-Vis spectrophotometer | Browning intensity measurement | Standard method for reaction kinetics |
Implications for Food and Biomedical Sciences
Understanding melanoidin formation and properties has significant implications across food science and biomedical research, influencing both technological applications and health outcomes.
In food systems, melanoidins contribute positively to sensory properties through color and flavor development but may also reduce nutritional value by decreasing protein bioavailability and essential amino acid content [1] [30]. Their antioxidant properties can improve food shelf-life, while their antibacterial activities against various foodborne pathogens offer potential for natural preservation [29]. Specific MRPs, including aminoreductones and glycated peptides, have demonstrated inhibitory effects against both Gram-positive and Gram-negative bacteria, including antibiotic-resistant strains such as methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant Pseudomonas aeruginosa [29].
In biomedical contexts, melanoidins and advanced glycation end-products (AGEs) formed in vivo have been implicated in chronic diseases including diabetes, cardiovascular disorders, and age-related conditions [11] [30]. However, certain MRPs also exhibit potential beneficial bioactivities, creating a complex risk-benefit profile that requires further investigation [30] [29]. The antibacterial properties of MRPs are being explored for medical applications, including medical device coatings and gut microbiota modulation, where they can prevent microbial colonization and influence host-microbe interactions [29].
The polymerization stage of the Maillard reaction, culminating in the formation of melanoidins and brown pigments, represents a complex interplay of chemical transformations that significantly influence the properties of processed foods and potentially human health. Despite advances in analytical methodologies, particularly with the application of FT-ICR-MS and other high-resolution techniques, the structural complexity of melanoidins continues to present substantial research challenges. Future research should focus on elucidating structure-activity relationships, optimizing reaction conditions to maximize beneficial properties while minimizing potentially harmful compounds, and developing standardized protocols for cross-study comparisons. The integration of multidisciplinary approaches combining advanced analytical techniques, bioactivity screening, and computational modeling will be essential to unravel the complexities of melanoidin chemistry and harness their potential in both food and biomedical applications.
The Maillard reaction represents one of the most complex reaction networks in food chemistry, traditionally characterized by reactions between amino groups from amino acids and carbonyl groups from reducing sugars. This technical review examines the S-Maillard reaction, a distinct pathway wherein thiolate groups from cysteine serve as nucleophilic participants rather than traditional amino groups. We explore the unique chemical mechanisms governing this pathway, its specialized reaction products, and the experimental methodologies essential for its investigation. Compared to classical Maillard pathways, the S-Maillard reaction generates different product profiles, including sulfur-containing conjugates and volatile compounds, while notably suppressing browning despite high product diversity. This comprehensive analysis provides researchers with advanced methodological frameworks for investigating thiolate-mediated pathways and their implications for flavor science, food safety, and therapeutic development.
Non-enzymatic browning represents a cornerstone reaction chemistry in food systems, primarily encompassing the Maillard reaction, caramelization, and oxidative pathways. The classical Maillard reaction is a complex network of reactions initiated between nucleophilic amino groups (primarily from amino acids, peptides, or proteins) and electrophilic carbonyl groups (primarily from reducing sugars) [11] [1]. This reaction cascade progresses through early, intermediate, and advanced stages, ultimately generating diverse compounds including aroma volatiles, pigments, and advanced glycation end-products (AGEs) [11] [31].
In contrast, the S-Maillard reaction constitutes a specialized pathway within this network, distinguished by the participation of thiolate groups (R-S¨−) as the primary nucleophiles [11]. These thiolate groups, primarily derived from cysteine residues in proteins or free cysteine, exhibit distinct reactivity patterns compared to amino groups. The term "S-Maillard" specifically differentiates reactions involving thiolate nucleophiles from those involving amino nucleophiles (classical Maillard) [11]. This distinction is mechanistically significant as the thiolate group possesses different nucleophilic strength, reaction kinetics, and product formation pathways compared to amino groups.
The physicochemical environment profoundly influences S-Maillard initiation. Thiolate groups (Cys; pKa ≈ 9) require specific pH conditions to exist in their deprotonated, nucleophilic form [11]. As the reaction medium's pH increases, the equilibrium shifts toward the nucleophilic thiolate form (RS−), enhancing its availability for S-Maillard pathways. This pH dependency creates a fundamental operational distinction from classical Maillard reactions involving lysine (Lys; pKa ≈ 10) or other amino groups [11].
Mechanistic Foundations of the S-Maillard Pathway
Initial Nucleophilic Addition
The S-Maillard reaction initiates with a nucleophilic addition wherein the thiolate sulfur attacks the electrophilic carbonyl carbon of a reducing sugar [11]. This elementary step generates a tetrahedral intermediate that serves as the pivotal branching point for subsequent transformations (Figure 1). The nucleophilic strength of the thiolate, governed by its relatively low pKa and high polarizability, often facilitates this addition more rapidly than comparable amino-group additions, particularly under moderate pH conditions [11].
The resulting thiocarbohydrate intermediates undergo diverse rearrangement and dehydration pathways distinct from those observed in classical Maillard reactions. These intermediates frequently lead to the formation of sulfur-containing heterocycles and cysteine-S-conjugates rather than the nitrogen-dominated heterocycles typical of amino-group Maillard pathways [32]. This divergent trajectory from the common intermediate fundamentally alters the product profile and sensory outcomes.
Intermediate Stage and Unique Product Formation
During the intermediate stage, the S-Maillard pathway generates characteristic sulfur-containing volatiles and non-volatile conjugates. Notably, the reaction between cysteine and pentoses (e.g., xylose, ribose) generates furfurylthiol (FFT) and 2-methyl-3-furanthiol (MFT), which are essential for the aroma of cooked meat [32]. These volatile thiols can further react to form non-volatile cysteine-S-conjugates (CS-conjugates) such as S-furfuryl-l-cysteine (FFT-S-Cys) and S-(2-methyl-3-furyl)-l-cysteine (MFT-S-Cys) [32].
The formation of these CS-conjugates represents a significant divergence from classical pathways. Research indicates that these conjugates may form through direct substitution reactions between cysteine and furfuryl derivatives, rather than through the classical Strecker degradation pathway [32]. These non-volatile CS-conjugates serve as flavor precursors that can be transformed into aroma-active thiols during consumption, often through enzymatic cleavage by oral microorganisms [32].
Table 1: Key Sulfur-Containing Compounds in S-Maillard Reactions
| Compound | Formation Pathway | Sensory Attributes | Significance |
|---|---|---|---|
| Furfurylthiol (FFT) | Cysteine + pentoses | Roasted coffee, cooked meat | Potent aroma compound |
| 2-Methyl-3-furanthiol (MFT) | Cysteine + pentoses | Meaty, savory | Character impact compound in meat |
| S-Furfuryl-l-cysteine (FFT-S-Cys) | Reaction of FFT with cysteine | Odorless precursor | In-mouth flavor release |
| S-(2-Methyl-3-furyl)-l-cysteine (MFT-S-Cys) | Reaction of MFT with cysteine | Odorless precursor | Potential flavor precursor |
Browning Suppression in S-Maillard Systems
A distinctive characteristic of cysteine-mediated Maillard reactions is their suppression of browning despite high reactivity and product diversity [6]. While lysine generates extensive browning and numerous reaction products, cysteine produces comparable molecular diversity with minimal pigment formation [6]. This phenomenon is attributed to cysteine's ability to form stable thiazolidine complexes and thioacetals that effectively trap reactive dicarbonyl intermediates, thereby diverting them from polymerization pathways that lead to melanoidin formation [6] [33].
The strategic application of cysteine or cysteine-rich ingredients can therefore function as a browning control mechanism in food processing, particularly in systems where color preservation is desirable without compromising flavor development potential.
Experimental Investigation of S-Maillard Pathways
Model System Design and Preparation
Investigating S-Maillard chemistry requires carefully controlled model systems. A foundational protocol involves reacting cysteine with xylose or ribose in aqueous solution, typically at concentrations of 0.1-0.2 M [6] [32]. These pentoses are preferentially selected due to their high reactivity compared to hexoses [6].
Standard Protocol: Cysteine-Xylose Model System
- Solution Preparation: Dissolve L-cysteine hydrochloride and D-xylose in phosphate buffer (75 mM, pH 7.4) to achieve 0.1 M final concentration each [32] [33].
- pH Adjustment: Adjust initial pH to specific value (e.g., 4.0-7.4) using NaOH or HCl [32]. Document precisely as pH significantly influences thiolate availability.
- Thermal Reaction: Transfer solutions to sealed vessels and heat at 100°C for 2-10 hours in temperature-controlled water bath or oven [6] [32].
- Reaction Termination: Cool samples rapidly in ice water and store at -20°C until analysis.
The reaction time and temperature should be optimized based on research objectives. Shorter times (2-4 hours) at 100°C favor intermediate product formation, while extended reactions (10+ hours) promote advanced products [6].
Advanced Analytical Characterization
Comprehensive S-Maillard product analysis requires multi-modal analytical approaches targeting both volatile and non-volatile compounds.
Volatile Compound Analysis:
- Technique: Gas Chromatography-Mass Spectrometry (GC-MS)
- Sample Preparation: Headspace solid-phase microextraction (HS-SPME) or simultaneous distillation extraction
- Key Targets: Furfurylthiol, 2-methyl-3-furanthiol, and other sulfur-containing volatiles [32]
Non-volatile Compound Analysis:
- Technique: Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR-MS)
- Conditions: Negative electrospray ionization (ESI-) for polar, oxygen-rich MRPs
- Data Processing: Molecular formula assignment via compositional networks and isotopic fine structure validation [6]
- Advantage: Ultrahigh resolution enables unambiguous detection of hundreds of distinct molecular formulae in complex MRPs [6]
Browning Assessment:
- Spectrophotometric Measurement: Absorbance at 294 nm (intermediate products) and 420 nm (advanced browning) [6] [33]
- Colorimetric Analysis: CIE Lab* color space measurements for visual color changes [31]
Table 2: Essential Research Reagent Solutions for S-Maillard Studies
| Reagent/Chemical | Function/Application | Technical Notes |
|---|---|---|
| L-Cysteine hydrochloride | Primary thiolate source | Highly hygroscopic; store under inert atmosphere |
| D-Xylose / D-Ribose | Reactive carbonyl source | Pentoses show higher reactivity than hexoses |
| Phosphate buffer (75 mM, pH 7.4) | Reaction medium | pH controls thiolate nucleophile availability |
| Deuterated solvents (D₂O, CD₃OD) | NMR analysis | For structure elucidation of novel conjugates |
| Derivatization reagents | GC-MS analysis | Thiol-specific derivatization enhances detection |
| HPLC-grade solvents | Chromatography | Acetonitrile/methanol with ammonium formate buffer |
Isolation and Identification of Cysteine-S-Conjugates
The characterization of novel CS-conjugates requires sophisticated isolation and structure elucidation protocols, as demonstrated for S-furfuryl-l-cysteine and S-(2-methyl-3-furyl)-l-cysteine [32]:
Isolation Protocol:
- Cation Exchange Chromatography: Initial fractionation of Maillard Reaction Product (MRP) on cation exchange resin
- Reversed-Phase Chromatography: Sub-fractionation on RP-18 column using water/ethanol gradient
- Semi-Preparative HPLC: Final purification using isocratic or shallow gradient elution
- Structure Elucidation: Nuclear Magnetic Resonance (NMR) spectroscopy ( [32]) and high-resolution MS for definitive structural characterization
Comparative Reaction Pathways: Classical vs. S-Maillard
The following diagram illustrates the distinct reaction pathways and products in classical Maillard versus S-Maillard reactions:
Research Implications and Future Directions
The S-Maillard reaction represents a fertile research domain with significant implications across multiple disciplines. In flavor science, the deliberate generation of cysteine-S-conjugates offers pathways to develop novel flavor precursors for controlled aroma release in processed foods [32]. For food safety, understanding cysteine's dual role in both forming and inhibiting potentially harmful compounds enables strategic intervention formulation [33]. In therapeutic development, elucidating the reaction mechanisms of thiol compounds with reactive carbonyls provides potential scaffolds for inhibiting advanced glycation end-product formation in vivo [11] [33].
Future research priorities should include:
- Structural Elucidation: Comprehensive characterization of novel CS-conjugates using hyphenated analytical techniques
- Kinetic Modeling: Quantitative assessment of reaction rates and pathways under varied physicochemical conditions
- Biological Activity Screening: Systematic evaluation of the toxicological and bioactive properties of S-Maillard products
- Process Optimization: Development of targeted strategies to promote desirable S-Maillard pathways while minimizing detrimental products
The S-Maillard reaction exemplifies how nuanced understanding of specific reaction pathways within broader chemical networks enables precise control over product outcomes, offering powerful tools for scientific and industrial innovation at the chemistry-biology interface.
Advanced Analysis and Biomedical Applications: From Model Systems to Human Health
Within the broader context of non-enzymatic browning and Maillard reaction research, the use of simplified model systems is a cornerstone methodology for deconstructing the profound complexity of these chemical pathways. The Maillard reaction, a non-enzymatic browning process between reducing sugars and amino compounds, generates a multitude of products (MRPs) that influence food quality, flavor, aroma, and color, and are also implicated in vivo in various diseases [6] [2]. Given that the reaction can produce thousands of distinct compounds from a few initial precursors, studying it in real food matrices is exceptionally challenging [6]. Ribose-amino acid model systems provide a controlled, reductionist approach to isolate and examine the specific reactions and variables that govern this intricate chemical network, thereby offering fundamental insights applicable across food science, nutrition, and biomedical fields [6] [34].
The Scientific Rationale for Ribose-Based Model Systems
The selection of ribose in model systems is a deliberate strategy grounded in its enhanced reactivity. As a pentose sugar, ribose undergoes Maillard reactions at a rate significantly faster than most common hexoses or sugar phosphates [6] [35]. This accelerated reaction kinetics allows researchers to observe a substantial progression of the reaction within experimentally feasible timeframes, even at moderate temperatures.
A key factor contributing to the high reactivity of ribose 5-phosphate is the strategic location of the 5-phosphate group near the reactive C1 center, which facilitates the reaction [35]. In fundamental proof-of-principle studies, unbuffered aqueous solutions containing ribose (0.1 M) and amino acids (0.1 M) incubated at 100°C for 10 hours generate a sufficient yield of MRPs for comprehensive analysis [6]. This high reactivity makes ribose an ideal candidate for probing the formation and structures of intermediate MRPs, which are often transient and difficult to capture in slower-reacting systems.
Experimental Protocols for Ribose-Amino Acid Model Systems
Standardized Model System Preparation
A foundational protocol for establishing a ribose-amino acid model system involves the following steps [6]:
- Solution Preparation: Prepare equimolar (0.1 M) aqueous solutions of D-ribose and the selected amino acid(s) in a solvent such as deionized water or a buffer like sodium phosphate (e.g., 0.2 M, pH 7.0) to control pH.
- Thermal Treatment: Pipette the reaction solution into sealed glass vials (e.g., 2 mL headspace vials). Heat the samples in a controlled dry bath or oven at a defined temperature, typically between 90°C and 100°C, for a set duration (e.g., 2, 4, 6, and 10 hours) [6].
- Reaction Termination: After heating, immediately cool the samples in an ice-water bath to quench the reaction.
- Sample Storage: Store the cooled samples at -20°C until analysis to prevent further reaction.
Monitoring Reaction Progress and Browning
The progression of the Maillard reaction can be tracked using simple spectrophotometric methods:
- Sample Dilution: Dilute the reacted sample appropriately with deionized water.
- Absorbance Measurement: Measure the absorbance of the diluted sample in a UV-Vis spectrophotometer at two key wavelengths:
- Colorimetric Analysis: Use a colorimeter to measure the CIE L* (lightness), a* (redness/greenness), and b* (yellowness/blueness) values. The total color difference (ΔE) can be calculated using the formula: ΔE = √[(L* - L₀)² + (a - a₀)² + (b - b₀)²] where L₀, a₀, and b₀ are the values of the unheated control sample [36].
Analytical Methodologies for Pathway Elucidation
Advanced analytical techniques are required to decode the complex reaction pathways.
Ultrahigh-Resolution Mass Spectrometry
Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR-MS) is a powerful tool for the non-targeted analysis of complex MRPs [6] [34].
- Sample Introduction: The reacted mixture is typically introduced via direct infusion after appropriate dilution with a compatible solvent (e.g., methanol/water).
- Ionization: Negative electrospray ionization (ESI(-)) is often used as it allows for the detection of polar and oxygen-rich MRPs from the initial and intermediate phases [6].
- Data Acquisition: The ultrahigh mass resolution and mass accuracy (< 1 ppm) of FT-ICR-MS enable the unambiguous assignment of molecular formulae to hundreds of distinct ion signals detected in a single analysis [6].
- Data Analysis: Van Krevelen diagrams (H/C vs. O/C atomic ratios) are used to visualize the data and identify common chemical transformations, such as dehydration, decarboxylation, and oxidation, by tracking the movement of data points on the plot [6].
Chromatographic Techniques for Specific Compounds
Liquid Chromatography (LC) coupled to mass spectrometry or UV detectors is employed for targeted analysis.
- Analysis of Sugars: The concentrations of precursor sugars (ribose, glucose, fructose) and their degradation products can be monitored using Gel Permeation Chromatography with a NH2 column and a refractive index detector. A mobile phase of acetonitrile and water (75:25 v/v) is commonly used [36].
- Analysis of α-Dicarbonyl Compounds: Key reactive intermediates like 3-deoxyglucosone (3-DG), glyoxal (GO), and methylglyoxal (MGO) can be quantified using LC after derivatization with specific reagents like o-phenylenediamine (OPD), followed by UV or MS detection [37] [36].
The following workflow diagram illustrates the integration of these protocols and methodologies in a typical study.
Key Findings from Ribose-Amino Acid Model Studies
Model system research has yielded critical quantitative data on the influence of amino acid side chains on Maillard reaction pathways.
Table 1: Reactivity and Product Formation in Ribose-Amino Acid Model Systems (10h at 100°C) [6]
| Amino Acid | Approximate Number of MRPs Detected | Relative Reactivity Order | Browning Intensity (A294 nm) |
|---|---|---|---|
| Lysine | > 700 | 1 (Highest) | Highest |
| Cysteine | 300 - 400 | 2 | Lowest |
| Isoleucine | 300 - 400 | 3 | Medium-High |
| Glycine | 300 - 400 | 3 | Medium |
Table 2: Impact of Amino Acid Side Chain on Reaction Pathways [6] [2] [38]
| Amino Acid | Reactive Group | Key Influence on Pathways and Products |
|---|---|---|
| Lysine | ε-Amino group (Primary amine) | High nucleophilicity leads to the greatest diversity of MRPs and strong browning; key in protein glycation. |
| Cysteine | Thiol group (-SH) | Participates in "S-Maillard" reactions; forms meat-like aromas and thiazolidines; suppresses browning but generates many MRPs. |
| Glycine | No side chain | Serves as a baseline for comparison; reactivity is primarily from the α-amino group. |
| Isoleucine | Aliphatic chain | Reactivity similar to glycine; side chain influences the formation of specific Strecker aldehydes (e.g., 2-methylbutanal). |
The Scientist's Toolkit: Essential Research Reagent Solutions
Table 3: Key Reagents and Materials for Ribose-Amino Acid Studies
| Reagent / Material | Function in Research | Example / Note |
|---|---|---|
| Reducing Sugars | Carbonyl group donor; primary reactant. | D-Ribose: Chosen for high reactivity. D-Glucose/D-Fructose: Common hexoses for comparison [6] [36]. |
| Amino Acids | Amino group donor; primary reactant. | Lysine, Cysteine, Glycine: Represent different side chain reactivities [6]. |
| Buffers | Control pH, a critical parameter influencing nucleophile availability and reaction kinetics. | Phosphate Buffer, Sodium Acetate Buffer [36]. |
| Analytical Standards | For identification and quantification of specific MRPs. | 5-HMF, Furfural, Glyoxal, Methylglyoxal, Nε-carboxymethyllysine (CML) [37] [36]. |
| Derivatization Reagents | To enhance detection of specific compounds. | o-Phenylenediamine (OPD): For α-dicarbonyl compound analysis [37]. |
| Solvents for Analysis | For sample dilution, separation, and MS ionization. | LC-MS Grade Methanol, Acetonitrile, Water. |
Ribose-amino acid model systems remain an indispensable tool in the chemist's arsenal for deconstructing the labyrinthine pathways of the Maillard reaction. The rigorous application of standardized experimental protocols, combined with advanced analytical techniques like FT-ICR-MS, allows researchers to generate reproducible and quantifiable data on reaction kinetics, product profiles, and the specific influence of reactant structures. The findings derived from these controlled systems provide the fundamental chemical understanding necessary to navigate the complexities of real-world food processing, storage, and even physiological glycation, ultimately informing strategies to optimize desirable outcomes and mitigate negative impacts across multiple industries.
The Maillard reaction, a complex network of non-enzymatic browning reactions between reducing sugars and amino compounds, represents one of the most intricate challenges in food chemistry and biomedical science. Since its discovery by Louis-Camille Maillard in 1912, this reaction has been recognized as the fundamental chemical process responsible for generating the appealing flavors, aromas, and colors in thermally processed foods, from roasted coffee to baked goods [39] [2]. Beyond its culinary significance, the Maillard reaction occurs in vivo and is implicated in the pathogenesis of diabetes mellitus, cardiovascular diseases, and other age-related disorders through the formation of advanced glycation end-products (AGEs) [39] [40]. The reaction progresses through three fundamental stages: the initial condensation forming Amadori rearrangement products, an intermediate phase with numerous degradation pathways, and final stages producing heterogeneous polymers known as melanoidins [39] [2].
The exceptional complexity of the Maillard reaction presents a formidable analytical challenge. Even simple model systems containing one sugar and one amino acid can generate thousands of distinct chemical compounds through interconnected reaction pathways [6] [41]. Traditional targeted analytical approaches, while valuable for studying specific marker compounds, inevitably miss the vast majority of reaction products, leaving critical gaps in our understanding of the complete reaction network [41] [42]. This limitation has hindered progress in controlling Maillard reaction outcomes for desired food qualities or mitigating its harmful effects in biological systems. Within this context, Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR-MS) has emerged as a powerful analytical technique capable of addressing this complexity through its unparalleled mass resolution and accuracy, enabling researchers to decode the molecular diversity hidden within the Maillard reaction with unprecedented detail [6] [41] [43].
Fundamentals of FT-ICR-MS Technology
Core Principles and Technical Specifications
Fourier Transform Ion Cyclotron Resonance Mass Spectrometry operates on the fundamental principle of ion cyclotron resonance, where ions trapped in a magnetic field move in circular paths with frequencies characteristic of their mass-to-charge ratios (m/z). When exposed to a radiofrequency electric field that matches their cyclotron frequency, these ions resonate, absorbing energy and moving in larger orbits. The resulting image current produced by these coherently moving ions is detected, digitized, and mathematically transformed using a Fourier transform to produce a mass spectrum of exceptional resolution and accuracy [43].
The extraordinary capabilities of FT-ICR-MS stem from several key technical advantages. The technique delivers ultrahigh mass resolution (>400,000 at m/z 300-400), enabling the separation of ions with minute mass differences that would be indistinguishable with conventional mass analyzers [6] [43]. This is complemented by exceptional mass accuracy (typically <0.2 ppm error), which allows for confident assignment of elemental compositions to detected ions [6]. Furthermore, FT-ICR-MS provides a wide dynamic range, capable of detecting compounds across approximately four orders of magnitude in concentration within complex mixtures—a crucial capability given the vast concentration ranges of Maillard reaction products (MRPs) [6].
Table 1: Key Technical Specifications of FT-ICR-MS in Maillard Reaction Studies
| Parameter | Typical Performance | Significance in Maillard Reaction Research |
|---|---|---|
| Mass Resolution | 400,000 at m/z 300-400 [6] [43] | Separates isobaric compounds with small mass differences |
| Mass Accuracy | <0.2 ppm average error [6] [43] | Enables confident elemental formula assignment |
| Mass Range | m/z 120-1000 [43] | Covers most MRPs from early to advanced stages |
| Dynamic Range | ~4 orders of magnitude [6] | Detects both abundant and trace MRPs in complex mixtures |
Comparative Advantages for Maillard Reaction Studies
The analytical power of FT-ICR-MS becomes particularly evident when compared to conventional mass spectrometry techniques in the context of Maillard reaction research. The extreme complexity of MRPs, often comprising thousands of compounds with similar elemental compositions, demands a platform capable of resolving this molecular complexity without prior chromatographic separation [41] [42]. While liquid chromatography coupled to mass spectrometry (LC-MS) provides valuable complementary information, the co-elution of numerous isomers and the limited resolution of conventional mass analyzers often prevents comprehensive molecular mapping [6].
FT-ICR-MS addresses these limitations through its ability to resolve and accurately mass-measure hundreds to thousands of compounds simultaneously in direct-infusion mode, without the need for chromatographic separation [6] [41]. This capability is paramount for non-targeted analysis, where the goal is to capture the entire molecular profile rather than focusing on predefined target compounds. The high mass accuracy ensures that each detected m/z value can be assigned to a unique molecular formula, providing a foundation for understanding reaction pathways and chemical transformations at a systems level [6] [43].
FT-ICR-MS in Action: Decoding Maillard Reaction Pathways
Molecular-Level Mapping of Reaction Networks
The application of FT-ICR-MS to Maillard model systems has revealed unprecedented insights into the staggering molecular diversity generated by these reactions. In a foundational study investigating ribose reacted with different amino acids, FT-ICR-MS detected more than 1,400 distinct molecular formulae from just four different model systems, with the number of MRPs following the reactivity order: lysine > cysteine > isoleucine ≈ glycine [6]. This extensive molecular mapping provided direct experimental evidence of how amino acid side chains influence reaction pathways, with lysine's nucleophilic ε-amino group and cysteine's thiol side chain leading to significantly higher MRP diversity compared to simpler amino acids [6].
Time-resolved studies of the ribose-glycine system further demonstrated the dynamic evolution of the Maillard reaction, with the number of MRPs increasing steadily over a 10-hour heating period at 100°C [41]. Notably, while traditional Maillard reaction schemes emphasize the degradation of the Amadori rearrangement product into smaller molecules, FT-ICR-MS analysis revealed the simultaneous formation of numerous compounds with higher molecular weight than the initial ARP, indicating that condensation and polymerization reactions occur concurrently with fragmentation pathways [41]. This challenges simplified representations of the Maillard reaction and underscores the need for analytical techniques that capture the full scope of reaction products.
Table 2: Molecular Diversity Detected by FT-ICR-MS in Maillard Model Systems
| Model System | Reaction Conditions | Number of MRPs Detected | Key Findings |
|---|---|---|---|
| Ribose-Lysine [6] | 10 h, 100°C | >700 MRPs | Highest reactivity due to nucleophilic side chain |
| Ribose-Cysteine [6] | 10 h, 100°C | 300-400 MRPs | Reactive thiol group enables diverse S-containing MRPs |
| Ribose-Glycine [6] [41] | 10 h, 100°C | 300-400 MRPs | ~348 MRPs after 10 h; linear increase over time |
| Ribose-Isoleucine [6] | 10 h, 100°C | 300-400 MRPs | Similar reactivity to glycine despite branched side chain |
Data Visualization and Interpretation Strategies
The immense datasets generated by FT-ICR-MS require sophisticated data visualization and analysis approaches to extract meaningful chemical insights. Van Krevelen diagrams have emerged as a powerful tool for visualizing complex molecular distributions based on elemental composition [6] [41]. These plots display the hydrogen-to-carbon (H/C) ratio against the oxygen-to-carbon (O/C) ratio for each detected molecular formula, creating a visual map where compounds with similar structural characteristics cluster together [41]. In Maillard reaction studies, van Krevelen diagrams effectively illustrate the progressive chemical transformations, with initial reactants located in specific regions (high H/C and O/C ratios) and reaction products shifting toward lower H/C and O/C values as dehydration and unsaturation increase over time [41].
Complementary to this approach, compositional network analysis based on exact mass differences reveals the chemical relationships between detected compounds [41] [42]. By identifying mass differences corresponding to known chemical transformations (e.g., dehydration: -18.01056 Da, decarboxylation: -43.98983 Da), researchers can reconstruct potential reaction pathways and identify key intermediates [41]. Studies have demonstrated that up to 98% of observed MRPs can be connected through just seven types of chemical transformations, highlighting the repetitive, modular nature of the Maillard reaction despite its overwhelming numerical complexity [41]. The molecular network approach effectively bridges the gap between non-targeted analysis and mechanistic chemistry, enabling hypothesis generation about reaction pathways directly from the comprehensive molecular data.
FT-ICR-MS Data Analysis Workflow
Experimental Protocols: FT-ICR-MS in Maillard Reaction Research
Standardized Workflow for Model System Analysis
The power of FT-ICR-MS for mapping Maillard reaction diversity is best realized through standardized experimental protocols that ensure reproducibility and meaningful data interpretation. A typical workflow begins with the preparation of model systems containing equimolar concentrations (e.g., 0.1 M) of a reducing sugar (such as ribose) and amino acid dissolved in appropriate buffers or water [6] [41]. These systems are heated at controlled temperatures (typically 80-100°C) for varying durations (2-10 hours) to capture different stages of the reaction progression. Parallel control samples containing only sugar or only amino acid heated under identical conditions are essential for distinguishing true MRPs from simple sugar degradation (caramelization) or amino acid decomposition products [41].
Following thermal treatment, samples require appropriate preparation for FT-ICR-MS analysis. For direct-infusion approaches, samples are typically diluted with suitable solvents (often methanol or water) and may undergo solid-phase extraction to remove salts and concentrate analytes of interest [43]. The exceptional sensitivity of FT-ICR-MS necessitates careful sample handling to avoid contamination, and the inclusion of quality control samples (e.g., pooled samples injected at regular intervals) is crucial for monitoring instrument performance throughout analytical batches [43]. For negative electrospray ionization (ESI-), which is particularly effective for detecting oxygen-rich MRPs prevalent in early and intermediate Maillard reaction stages, samples are often acidified to enhance ionization efficiency [6] [41].
Critical Measurement Parameters and Data Processing
FT-ICR-MS measurements for Maillard reaction analysis demand careful optimization of several key parameters. The ultrahigh mass resolution (≥400,000 at m/z 300-400) enables separation of isobaric compounds with minute mass differences, while high mass accuracy (typically requiring <0.5 ppm error, often <0.2 ppm) is essential for confident molecular formula assignment [6] [43]. Internal calibration using known reference compounds or an in-house calibration list of masses abundant in the sample type is typically employed to maintain mass accuracy throughout extended measurement sequences [43].
Data processing represents a critical step in transforming raw mass spectra into chemically meaningful information. This typically involves peak picking, internal calibration, and formula assignment using algorithms that consider possible combinations of carbon, hydrogen, oxygen, nitrogen, and sulfur atoms [6] [43]. Advanced strategies incorporate isotopic fine structure validation to eliminate false formula assignments, leveraging the fact that the relative abundances of isotopic peaks (e.g., ¹³C, ³⁴S) must match the theoretical distribution for the proposed formula [6]. For robust results, molecular features are typically required to be present in multiple analytical replicates, with intensity thresholds applied to distinguish true signals from background noise [43].
Table 3: Essential Research Reagent Solutions for FT-ICR-MS Maillard Studies
| Reagent/Material | Specifications | Function in Experimental Protocol |
|---|---|---|
| Reducing Sugars | High-purity (e.g., D-ribose, D-glucose) | Primary carbonyl reactant in Maillard model systems |
| Amino Acids | ACS grade or higher (e.g., glycine, lysine, cysteine) | Primary amino reactant; side chains influence pathways |
| Solvents | LC-MS grade water, methanol, acetonitrile | Sample preparation, dilution, and mobile phases |
| Solid-Phase Extraction Cartridges | C18 or mixed-mode sorbents | Desalting and concentration of MRPs prior to analysis |
| Internal Calibration Standards | Known compounds or custom mass lists | Maintaining sub-ppm mass accuracy during FT-ICR-MS |
| Mobile Phase Additives | LC-MS grade ammonium acetate, formic acid | Enhancing ionization efficiency in ESI |
Applications in Complex Food Systems and Biomedical Research
Beyond Model Systems: Monitoring Industrial Processes
While model systems provide fundamental insights, FT-ICR-MS truly demonstrates its utility when applied to complex real-world samples. In brewing science, researchers have employed FT-ICR-MS to track molecular fluctuations throughout the entire brewing process, from raw barley to finished beer [43] [42]. This approach revealed over 8,000 unique molecular descriptors during brewing, enabling the identification of malt-specific molecular imprints that persist through various process stages [43]. By comparing beers brewed with malts kilned at different temperatures (80°C for Pilsner malt vs. 100°C for Munich malt), researchers could directly observe the impact of Maillard reaction intensity on the final molecular composition, linking traditional brewing attributes (EBC color value, free amino nitrogen) to specific molecular patterns [43].
Similar approaches have been applied to study the molecular diversity of commercial beer products. Analysis of 250 beer samples from diverse styles and geographical origins demonstrated that non-targeted FT-ICR-MS profiling could capture the substantial contribution of Maillard reaction products to the overall beer metabolome [42]. Molecular networks derived from the accurate mass data revealed characteristic patterns associated with different beer types and brewing processes, highlighting the potential for quality control and authentication applications [42]. These studies demonstrate how FT-ICR-MS bridges the gap between simplified model systems and the complexity of real food products, providing actionable insights for process optimization and quality assurance.
Implications for Health and Disease
The analytical power of FT-ICR-MS extends beyond food science to biomedical research, where Maillard reaction products formed in vivo (AGEs) are increasingly recognized as important players in disease pathogenesis. The comprehensive molecular mapping enabled by FT-ICR-MS provides unprecedented opportunities to identify and characterize these biologically relevant compounds [39] [40]. In diabetes research, for example, FT-ICR-MS could potentially identify novel protein modifications resulting from non-enzymatic glycation, expanding our understanding of how hyperglycemia leads to cellular dysfunction [39].
Recent studies have begun to explore the health implications of dietary melanoidins, the high molecular weight MRPs that escape digestion and reach the colon intact [44]. There is growing evidence that these compounds may function as prebiotic-like substances, undergoing microbial fermentation to produce short-chain fatty acids that support gut barrier function and modulate inflammation [44]. FT-ICR-MS, with its ability to characterize complex molecular mixtures, is ideally positioned to elucidate the structural features of melanoidins that influence their fermentation by gut microbiota and subsequent bioactivities, potentially opening new avenues for functional food development and personalized nutrition strategies [44].
Future Perspectives and Concluding Remarks
FT-ICR-MS has unequivocally established itself as a transformative analytical technique for mapping the molecular diversity of the Maillard reaction, providing insights that were previously inaccessible through conventional targeted approaches. The ability to resolve thousands of compounds simultaneously, assign elemental compositions with high confidence, and visualize complex molecular relationships has fundamentally advanced our understanding of this quintessential reaction network. As the technology continues to evolve, several promising directions emerge for future research.
The integration of FT-ICR-MS with separation techniques such as liquid chromatography or ion mobility spectrometry represents a powerful approach to address the inherent limitation of isomer discrimination in direct-infusion MS [42]. While FT-ICR-MS excels at determining elemental compositions, it cannot distinguish between structural isomers without additional separation dimensions. Complementary techniques such as nuclear magnetic resonance (NMR) spectroscopy and tandem mass spectrometry will be essential for structural elucidation of key MRPs identified through FT-ICR-MS screening [45].
From an applications perspective, the translation of molecular-level knowledge into process control strategies represents a crucial frontier. In industrial contexts such as food processing and biopharmaceutical manufacturing, where the Maillard reaction impacts product quality, stability, and safety, FT-ICR-MS-derived molecular signatures could inform real-time process analytics and control systems [39] [45]. The development of streamlined data processing pipelines and standardized reporting frameworks will be essential to maximize the impact of FT-ICR-MS in both research and industrial settings.
In conclusion, FT-ICR-MS has emerged as an indispensable tool for deciphering the complex molecular landscape of the Maillard reaction. By providing a comprehensive view of the reaction network that transcends the limitations of targeted approaches, this powerful analytical technique enables researchers to connect chemical structures to reaction pathways, process conditions, and ultimately functional properties—paving the way for optimized food quality, enhanced product stability, and improved understanding of the health implications of Maillard chemistry.
Non-enzymatic browning (NEB), primarily through the Maillard reaction and caramelization, represents a complex set of chemical transformations that occur during the thermal processing and storage of foods. These reactions are critically important in food science, responsible for the development of desirable aromas, flavors, and colors in everything from baked goods and roasted coffee to cooked meats [1] [46]. The Maillard reaction, first described by Louis-Camille Maillard in 1912, occurs between carbonyl groups of reducing sugars and nucleophilic amino groups of amino acids, peptides, or proteins [2] [47]. This cascade of chemical events progresses through initial, intermediate, and advanced stages, generating a multitude of chemical compounds with varying sensory and biological activities [1] [2].
Beyond its culinary significance, the Maillard reaction has profound implications for human health and disease pathology. When these reactions occur in vivo under physiological conditions, they lead to the formation of advanced glycation end-products (AGEs) through non-enzymatic modification of biological macromolecules including proteins, lipids, and nucleic acids [48]. These modifications can alter the structure and functional activity of affected molecules, contributing to physiologic aging and various disease processes [48]. The detection and quantification of specific biomarkers generated during non-enzymatic browning reactions thus serve dual purposes: monitoring food quality and processing conditions, while also understanding their potential impact on human health.
Among the plethora of compounds formed, three classes of biomarkers have emerged as particularly significant for analytical monitoring: 5-hydroxymethylfurfural (HMF), furfural, and AGEs. HMF and furfural are intermediate products formed during sugar degradation, while AGEs represent heterogeneous compounds formed in the advanced stages of the Maillard reaction [1] [49] [47]. This technical guide provides an in-depth examination of these critical biomarkers, their analytical quantification, formation pathways, and significance within food and biomedical research.
Key Biomarkers: Formation Pathways and Significance
5-Hydroxymethylfurfural (HMF) and Furfural
5-Hydroxymethylfurfural (HMF) is a heterocyclic organic compound (C6H6O3) that serves as a crucial marker of thermal processing and food quality [50]. It forms through the dehydration of hexose sugars under acidic conditions during caramelization, or through the Maillard reaction between reducing sugars and amino compounds [50] [49]. HMF has garnered significant attention due to its association with potential health concerns, including mucosal toxicity, cytotoxicity, neurotoxic effects, and DNA damage [50]. Its metabolite, 5-sulfooxymethylfurfural (5-SMF), has been shown to react with DNA, producing different DNA adducts and causing mutagenic effects [51]. Despite these concerns, some research indicates potential beneficial effects at lower concentrations, such as antioxidant and anti-allergic properties [52].
Furfural is a related compound that forms from pentose sugars through similar dehydration pathways. While less extensively studied than HMF, it serves as an important indicator of browning reactions, particularly in systems rich in pentose sugars.
The presence of HMF in foods varies significantly based on processing conditions and food matrix. Coffee contains particularly high levels (100-1000 mg/kg) due to intensive roasting, while honey typically shows concentrations between 10-200 mg/kg, influenced by storage duration and temperature [52]. Bread and baked goods generally exhibit lower levels (1-50 mg/kg), though these can increase significantly with intense thermal treatment or specific ingredient combinations [52]. In traditional Iranian breads, for example, HMF concentrations ranged from 15.46 mg/kg in Taftoon to 74.32 mg/kg in Sangak bread [52].
Regulatory authorities have established limits for HMF in certain food categories. The Codex Alimentarius Commission and the European Union have set maximum permissible levels of HMF in honey and apple juice at 40 mg/kg and 50 mg/kg, respectively [50]. For caramel colors (E150a-d), while no maximum values are currently set for HMF, specifications exist for other heat-derived contaminants like 4-methylimidazole (4-MI) [51].
Table 1: Occurrence of HMF in Selected Food Products
| Food Product | Typical HMF Concentration Range | Key Influencing Factors |
|---|---|---|
| Coffee | 100-1000 mg/kg | Roasting intensity, bean type |
| Honey | 10-200 mg/kg | Storage time, temperature, floral source |
| Bread & Baked Goods | 1-50 mg/kg | Baking conditions, ingredients, pH |
| Caramel Colors | 10-33,700 mg/kg | Production time, temperature, class type |
| Fruit Juices | 5-100 mg/kg | Processing, storage, concentration |
| Dried Fruits | 10-500 mg/kg | Dehydration method, temperature |
Advanced Glycation End-products (AGEs)
Advanced glycation end-products (AGEs) represent a heterogeneous group of compounds formed in the advanced stages of the Maillard reaction [48]. These compounds result from non-enzymatic covalent modification of macromolecules such as proteins, lipids, and nucleic acids by reducing sugars [48]. The formation of AGEs occurs through complex chemical pathways that involve rearrangements, dehydrations, oxidations, and cross-linking reactions [1] [47].
AGEs have been implicated in numerous age-related pathological conditions. In physiological aging, AGEs contribute to age-dependent covalent cross-linking and modification of proteins in connective tissues such as eyes and skin [48]. Collagen, with its long half-life, is particularly susceptible to AGE accumulation, leading to decreased elasticity and functionality. Pathological processes associated with AGEs include autoimmune and inflammatory diseases, neurodegenerative diseases (Alzheimer's and Parkinson's), diabetes and diabetic nephropathy, cardiovascular diseases, and bone degenerative diseases [48].
The measurement of AGEs presents significant analytical challenges due to their heterogeneous nature. Common AGE biomarkers include N(6)-carboxymethyllysine (CML), which has been widely studied as a marker of protein glycation [1] [2]. Other significant AGEs include pentosidine, methylglyoxal-derived hydroimidazolone, and glyoxal-derived lysine dimer.
Table 2: Key Advanced Glycation End-products (AGEs) and Their Significance
| AGE Compound | Formation Pathway | Biological Significance |
|---|---|---|
| N(6)-carboxymethyllysine (CML) | Glyoxal-mediated modification of lysine residues | Most widely studied AGE biomarker; correlates with diabetic complications |
| Pentosidine | Cross-linking AGE from pentose sugars | Fluorescent cross-link; marker of tissue aging and diabetes |
| Methylglyoxal-derived hydroimidazolone (MG-H1) | Reaction of methylglyoxal with arginine residues | Major antigenic AGE; implicated in diabetic complications |
| Glyoxal-derived lysine dimer (GOLD) | Cross-linking reaction between two lysine residues | Contributes to protein cross-linking and tissue stiffness |
Analytical Methodologies for Biomarker Quantification
Analytical Techniques for HMF and Furfural
The quantification of HMF and furfural employs a diverse range of analytical techniques, each with distinct advantages and limitations. Spectrophotometric methods provide a rapid, straightforward approach for assessing browning intensity through absorbance measurements at fixed wavelengths (typically 284 nm for HMF and 280 nm for furfural) [1]. While simple and cost-effective, these methods lack specificity as they measure total absorbance without distinguishing between individual compounds.
Chromatographic techniques offer significantly improved specificity and sensitivity. High-performance liquid chromatography (HPLC) with UV detection represents the most widely applied method for HMF quantification in various food matrices [1] [52]. This approach provides excellent separation capabilities and can simultaneously quantify multiple analytes. For enhanced sensitivity and structural confirmation, liquid chromatography-mass spectrometry (LC-MS) has become the gold standard, particularly when coupled with stable isotope-labeled internal standards such as HMF-13C6 to compensate for matrix effects and ionization variability [50] [51].
Recent methodological advances have focused on increasing throughput and sustainability. Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) with in-situ derivatization using 5,10,15,20-tetrakis-(4-aminophenyl)-porphyrin (TAPP) has been developed as a rapid, high-throughput approach for HMF analysis [50]. This method shifts the molecular weight of HMF derivatives to higher m/z regions (m/z 782.68 for TAPP-HMF), effectively eliminating interference from the organic matrix and achieving detection limits of 0.347 mg/kg with outstanding linearity [50].
For furfural analysis, gas chromatography-mass spectrometry (GC-MS) is frequently employed due to the volatile nature of furfural and its derivatives [1]. This technique provides excellent separation efficiency and sensitivity for volatile Maillard reaction products.
Table 3: Analytical Techniques for HMF and Furfural Quantification
| Analytical Technique | Key Features | Limitations | Reported LOD/LOQ |
|---|---|---|---|
| Spectrophotometry | Rapid, cost-effective, simple sample preparation | Lacks specificity, measures total absorbance | Varies by matrix |
| HPLC-UV | Excellent separation, widely accessible, quantitative | Limited structural confirmation, matrix interference | LOQ: 5 ng/mL for liquid samples [51] |
| LC-MS/MS | High specificity and sensitivity, structural confirmation | Higher cost, requires expertise, matrix effects | LOD: 7.2 mg/kg, LOQ: 21 mg/kg for caramel colors [51] |
| MALDI-MS with derivatization | High-throughput, minimal sample needs, green chemistry | Specialized equipment, derivatization optimization | LOD: 0.347 mg/kg for honey [50] |
| GC-MS | Ideal for volatile compounds, high sensitivity | Requires volatility, sample derivatization often needed | Varies by specific method |
Analytical Techniques for AGEs
The analysis of AGEs presents unique challenges due to their heterogeneous nature and the complexity of biological and food matrices. Immunoassays, particularly enzyme-linked immunosorbent assays (ELISAs), were historically employed for AGE detection but have limitations in specificity and cross-reactivity in complex matrices [1].
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) has emerged as the preferred methodology for specific AGE quantification, offering high sensitivity and structural specificity [1]. This technique enables the precise measurement of individual AGE structures such as CML, pentosidine, and MG-H1 in both foods and biological samples. Sample preparation typically involves protein hydrolysis followed by solid-phase extraction cleanup to remove interfering compounds.
Fluorescence spectroscopy provides a complementary approach for detecting fluorescent AGEs like pentosidine, while non-targeted analysis using Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) offers unprecedented insights into the immense chemodiversity of Maillard reaction products, with studies identifying more than 1400 different molecular formulae in ribose-amino acid model systems [6].
Experimental Protocols and Workflows
Sample Preparation and Extraction Methods
Effective sample preparation is critical for accurate quantification of browning biomarkers. For HMF analysis in bread matrices, Method-B (adapted from Gülcan et al.) has demonstrated superior sustainability and cost-effectiveness, achieving an AGREE score of 0.52 in green chemistry assessments [52]. This method utilizes minimal sample (0.5 g) and Carrez reagent volumes with semi-automated homogenization, reducing hazardous waste and material expenses while maintaining analytical performance.
For HMF extraction from honey, a green approach using ethyl acetate extraction followed by derivatization with TAPP (5,10,15,20-tetrakis-(4-aminophenyl)-porphyrin) enables sensitive MALDI-MS detection [50]. The derivatization reaction proceeds quickly at room temperature without requiring catalyst addition, simplifying the workflow while maintaining high sensitivity.
For AGE analysis from biological tissues, samples typically undergo protein hydrolysis using 6M HCl at 110°C for 16-24 hours, followed by solid-phase extraction cleanup using C18 or mixed-mode cartridges. Internal standards such as 13C-labeled CML or d4-lysine are essential for accurate quantification due to matrix effects and potential losses during sample preparation.
HMF Analysis Workflow Using MALDI-MS with Derivatization
The following workflow illustrates the optimized procedure for high-throughput HMF analysis in honey samples using MALDI-MS with in-situ derivatization [50]:
Diagram 1: HMF Analysis Workflow
Method Validation Parameters
Robust method validation is essential for generating reliable quantitative data. Key validation parameters include:
- Linearity: assessed through calibration curves with correlation coefficients (R²). The TAPP-based MALDI-MS method demonstrated outstanding linearity for HMF quantification [50].
- Limit of Detection (LOD) and Quantification (LOQ): For HMF in caramel colors, LOD and LOQ values of 7.2 mg/kg and 21 mg/kg, respectively, have been reported using LC-MS methods [51].
- Precision: evaluated through intra-day and inter-day relative standard deviation (RSD). Acceptable precision typically requires RSD values <10-15%.
- Accuracy: determined through recovery studies at multiple concentration levels. Recovery rates of 100-105% have been reported for HMF in caramel colors [51].
- Specificity: ensured through chromatographic separation or high-resolution mass spectrometry to distinguish target analytes from matrix interferences.
Health Implications and Risk Assessment
Toxicological Considerations
The potential health implications of browning biomarkers, particularly HMF and AGEs, have garnered significant scientific and regulatory attention. For HMF, toxicological concerns center on its metabolic conversion to 5-sulfooxymethylfurfural (5-SMF), a reactive intermediate that can form DNA adducts and exhibit genotoxic potential in vitro [51] [52]. Animal studies have indicated that high doses of HMF can initiate and promote colon cancer and cause renal tubular hyperplasia and hepatotoxicity, though these effects occur at exposure levels far exceeding typical dietary intake [52].
AGEs contribute to pathological processes through multiple mechanisms, including protein cross-linking that alters tissue structure and function, receptor for AGE (RAGE) activation that triggers pro-inflammatory signaling pathways, and oxidative stress generation through interaction with cellular components [48]. The accumulation of AGEs in tissues has been correlated with the progression of diabetes complications, cardiovascular disease, neurodegenerative disorders, and physiological aging [48].
Exposure Assessment and Risk Characterization
Comprehensive exposure assessments have been conducted to evaluate potential health risks associated with browning biomarkers. For HMF, the European Food Safety Authority (EFSA) has concluded that estimated daily intake for European consumers is generally low and does not pose significant health concerns, with mean adult exposure around 0.3 mg/kg body weight per day [52]. However, specific population subgroups, particularly children consuming certain food categories like fruit juices, may experience higher exposure levels warranting continued monitoring.
Risk assessment metrics including Target Hazard Quotient (THQ), Incremental Lifetime Cancer Risk (ILCR), and Margin of Exposure (MOE) provide quantitative frameworks for evaluating potential health risks. For traditional Iranian breads, THQ values for all age groups were below 1, indicating low immediate non-carcinogenic risk from HMF exposure [52]. However, uncertainty analysis suggested potential carcinogenic risks for all age groups consuming certain bread types, particularly Sangak bread [52].
For caramel colors, exposure assessments for Danish adolescents and adults indicate that 5-HMF exposure from E150a, E150c, and E150d caramel colors remains below threshold levels of concern, though monitoring is recommended due to variations in production methods and resulting HMF concentrations [51].
Table 4: Risk Assessment Metrics for HMF Exposure from Traditional Iranian Breads [52]
| Bread Type | Age Group | THQ Value | Cancer Risk Assessment |
|---|---|---|---|
| Sangak | Children | <1 | Potential carcinogenic risk |
| Sangak | Adolescents | <1 | Potential carcinogenic risk |
| Sangak | Adults | <1 | Potential carcinogenic risk |
| Barbari | Children | <1 | Potential carcinogenic risk |
| Taftoon | All age groups | <1 | Lower risk profile |
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful analysis of browning biomarkers requires carefully selected reagents, reference standards, and specialized materials. The following toolkit summarizes essential components for research in this field:
Table 5: Research Reagent Solutions for Browning Biomarker Analysis
| Reagent/Material | Specifications | Application Purpose |
|---|---|---|
| HMF Standard | >99.9% purity, with 13C6-labeled internal standard | Quantification standard for LC-MS/MS and calibration |
| TAPP Derivatization Agent | 5,10,15,20-Tetrakis-(4-aminophenyl)-porphyrin, ≥95% | MALDI-MS derivatization to enhance HMF detection sensitivity |
| CML Standard | N(6)-Carboxymethyllysine, with 13C or d4-labeled internal standard | AGE quantification reference standard |
| Carrez Reagents | Potassium hexacyanoferrate(II) and zinc sulfate | Protein precipitation and clarification in food extracts |
| Solid-Phase Extraction Cartridges | C18, mixed-mode, or specialized sorbents | Sample cleanup and concentration prior to analysis |
| MALDI Matrices | CHCA (α-cyano-4-hydroxycinnamic acid), purified | Matrix for laser desorption/ionization in MS analysis |
| Acid Hydrolysis Reagents | 6M HCl, nitrogen-scrubbed, sequencing grade | Protein hydrolysis for total AGE analysis |
| Enzymatic Digestion Kits | Protease cocktails (e.g., pronase, proteinase K) | Enzymatic release of protein-bound AGEs |
The quantitative analysis of HMF, furfural, and AGEs as biomarkers of non-enzymatic browning provides critical insights into both food quality and potential health implications. Advanced analytical methodologies, particularly mass spectrometry-based approaches, continue to evolve toward higher sensitivity, throughput, and sustainability. The ongoing characterization of formation pathways and biological effects of these compounds remains essential for developing mitigation strategies and guiding regulatory decisions. As research progresses, the integration of non-targeted analytical approaches with mechanistic toxicology studies will further elucidate the complex relationship between dietary browning biomarkers and human health, enabling evidence-based recommendations for food processing and consumption.
The Maillard reaction, first described by Louis-Camille Maillard in 1912, is a chemical reaction between amino acids and reducing sugars that gives browned food its distinctive flavor and color [30] [2]. For decades, this reaction was primarily the concern of food chemists. However, a pivotal discovery in 1968 revealed that the same non-enzymatic browning reaction occurs in vivo, with the identification of a glucose-modified hemoglobin (HbA1c) in diabetic patients [53]. This finding established that Maillard chemistry is not confined to food processing but is a critical physiological and pathological process in living organisms.
When this complex network of reactions occurs in the body, it is termed glycation—a spontaneous, non-enzymatic modification of proteins, lipids, and nucleic acids by reducing sugars and related metabolites [53] [54]. The final products of this process, known as advanced glycation end products (AGEs), are now recognized as key players in the physiology of aging and the pathogenesis of numerous age-related diseases, particularly diabetes mellitus and its complications [55] [48]. This review explores the molecular mechanisms linking Maillard chemistry to human disease, with a focus on protein glycation in diabetes and aging, providing researchers and drug development professionals with a comprehensive technical guide to this critical field.
Molecular Mechanisms of Protein Glycation
The Glycation Cascade: From Initial Encounter to Advanced Products
The process of protein glycation is a complex cascade that progresses through several stages, each characterized by increasingly stable and potentially damaging products.
Initial Stage (Early Glycation): The process begins when the electrophilic carbonyl group of a reducing sugar (primarily glucose in vivo) reacts with a nucleophilic amino group on a protein—typically the ε-amino group of lysine residues or the guanidino group of arginine residues [2] [54]. This forms a reversible Schiff base. The Schiff base then undergoes an Amadori rearrangement to form a more stable, but still reversible, Amadori product (e.g., fructosamine on proteins or HbA1c on hemoglobin) [56] [53]. These early glycation adducts can serve as biomarkers for medium-term glycemic control.
Intermediate Stage: Amadori products undergo further reactions, including dehydration, fragmentation, and oxidation, leading to the formation of highly reactive α-dicarbonyl compounds (also known as dicarbonyls) [57]. The most physiologically relevant dicarbonyls are methylglyoxal (MGO), glyoxal (GO), and 3-deoxyglucosone (3-DG) [58] [56]. These compounds are far more reactive than glucose itself and are major precursors to AGEs.
Advanced Stage: The reactive dicarbonyl compounds react irreversibly with amino groups, particularly on lysine and arginine residues, to form a heterogeneous group of stable compounds known as advanced glycation end products (AGEs) [53] [57]. This stage also involves oxidative reactions, often termed "glycoxidation" [56].
The following diagram illustrates the key stages and pathways of the AGE formation process:
Key Advanced Glycation End Products (AGEs)
More than 20 different AGEs have been identified in human tissues [57]. They are classified based on their fluorescence and cross-linking properties, which directly influence their pathological impact.
Table 1: Key Advanced Glycation End Products (AGEs) and Their Characteristics
| AGE Name | Precursor | Fluorescence | Cross-linking | Biological Impact |
|---|---|---|---|---|
| CML(Nε-carboxymethyl-lysine) | Glyoxal, Amadori products | No | No | Marker of oxidative stress; modifies protein charge and function [55] [56] |
| CEL(Nε-carboxyethyl-lysine) | Methylglyoxal | No | No | Indicator of dicarbonyl stress; correlates with metabolic dysfunction [58] [56] |
| MG-H1(Methylglyoxal-derived hydroimidazolone) | Methylglyoxal | No | No | Most abundant AGE in vivo; alters protein function [58] [56] |
| Pentosidine | Pentose sugars | Yes | Yes | Classic cross-link; used as a biomarker for aging and disease [55] [57] |
| Glucosepane | Glucose | Yes | Yes | Most abundant cross-linking AGE in human tissue; major role in tissue stiffening [56] |
The AGE-RAGE Signaling Axis: A Pathway to Pathology
The detrimental effects of AGEs are largely mediated through their interaction with a specific cellular receptor, the Receptor for AGE (RAGE). RAGE is a multi-ligand, pattern recognition receptor belonging to the immunoglobulin superfamily [55]. The binding of AGEs to RAGE, particularly its full-length, membrane-bound form, initiates a robust and sustained pro-inflammatory signaling cascade.
- Key Signaling Pathways: AGE-RAGE binding activates several critical pathways, most notably the NF-κB (Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B cells) pathway. This activation leads to the increased transcription of genes encoding pro-inflammatory cytokines (e.g., TNF-α, IL-1, IL-6), adhesion molecules, and RAGE itself, creating a positive feedback loop that perpetuates cellular stress [55]. Concurrently, AGE-RAGE interaction activates NADPH oxidase, leading to a rapid and significant burst of Reactive Oxygen Species (ROS) [55] [57]. This oxidative stress further accelerates AGE formation and damages cellular components, creating a vicious cycle of glycation and inflammation.
The central role of the AGE-RAGE axis in disease pathology is visualized in the following pathway diagram:
Glycation as a Core Mechanism in Diabetes and Aging
Glycation in Diabetes Mellitus: Beyond Hyperglycemia
In diabetes mellitus, persistent hyperglycemia provides an abundant supply of glucose, dramatically accelerating the glycation process [54]. This elevated glycation burden is a primary driver of the microvascular and macrovascular complications that characterize the disease.
Established Glycation Biomarkers: The measurement of glycated hemoglobin (HbA1c) is the gold-standard clinical biomarker for assessing long-term (2-3 months) glycemic control in diabetic patients [56] [54]. Similarly, glycated albumin (GA) reflects shorter-term (2-3 weeks) glycemic control and is particularly useful in situations where HbA1c may be unreliable, such as in patients with hemoglobinopathies or chronic kidney disease [56] [54].
Dicarbonyl Stress: A critical consequence of hyperglycemia is the increased formation of reactive dicarbonyls, primarily methylglyoxal (MGO). MGO is formed mainly as a byproduct of glycolysis and is a potent arginine-directed glycating agent [56] [54]. Under diabetic conditions, the body's defense systems for detoxifying MGO (e.g., the glyoxalase system) can become overwhelmed, leading to a state of "dicarbonyl stress" [56]. This stress results in the rapid formation of AGEs like MG-H1, which are directly implicated in the dysfunction of vascular endothelial cells, podocytes, and pancreatic beta-cells [54].
Glycation in the Aging Process: The "Common Soil"
The accumulation of AGEs is a hallmark of the normal aging process, often referred to as "physiologic aging" [48] [57]. Long-lived structural proteins with low turnover rates, such as collagen in the skin, blood vessels, and connective tissues, and crystallins in the eye lens, are particularly susceptible to cumulative AGE modification over a lifetime [55] [57].
Tissue Stiffening and Dysfunction: The progressive formation of AGE cross-links on collagen and elastin fibers contributes directly to age-related tissue stiffening. This leads to a loss of elasticity in the skin (wrinkling) and arteries (increased vascular stiffness and systolic hypertension) [57]. In the lens of the eye, cross-linking of crystallins causes lens opacification, contributing to cataract formation [57].
The "Common Soil" Hypothesis: AGEs are increasingly seen as a "common soil" linking normal aging with age-related diseases like diabetes, cardiovascular disease, and neurodegeneration [55] [48]. The mechanisms of oxidative stress, chronic inflammation, and cumulative macromolecular damage driven by the AGE-RAGE axis are shared across these conditions, with the rate of AGE accumulation being accelerated by pathological states like hyperglycemia.
Analytical Methods and Experimental Protocols
Accurate measurement of glycation adducts is essential for both clinical diagnostics and research. The methodologies vary in complexity, sensitivity, and the specific information they provide.
Table 2: Key Methodologies for Measuring Protein Glycation Adducts
| Method | Target Analytes | Principle | Advantages | Limitations |
|---|---|---|---|---|
| Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) | Protein-bound and free adducts of CML, CEL, MG-H1, G-H1, etc. [56] | Chromatographic separation followed by mass-based detection and quantification using stable isotopic internal standards. | High specificity and sensitivity; can quantify multiple analytes simultaneously (multiplexing); gold standard for research [56]. | Requires expensive instrumentation and expert operation; complex sample preparation. |
| Skin Autofluorescence (SAF) | Total fluorescent AGEs (e.g., Pentosidine) in the skin [58] [55] | Measurement of the fluorescence emitted by certain AGEs in the skin when excited by UV-A light. | Non-invasive, rapid, and provides a long-term index of AGE accumulation; good for large-scale screening [55]. | Measures only fluorescent AGEs; results can be influenced by skin pigmentation and other chromophores. |
| Enzyme-Linked Immunosorbent Assay (ELISA) | Specific AGEs (e.g., CML, Pentosidine) | Use of antibodies specific to a particular AGE structure for colorimetric detection. | High throughput; relatively low cost; suitable for clinical samples [57]. | Potential for antibody cross-reactivity; less specific and absolute than LC-MS/MS. |
| Ion-Exchange Chromatography / HPLC | HbA1c [54] | Separation of hemoglobin variants based on charge differences caused by glycation. | Well-established, automated, and standardized for clinical use with HbA1c. | Primarily limited to HbA1c analysis. |
Detailed Experimental Protocol: Quantification of Serum AGEs using LC-MS/MS
The following protocol outlines the standard procedure for the precise quantification of major serum AGEs, based on established methodologies in the field [56].
I. Sample Preparation
- Aliquoting: Pipette 50 µL of serum or plasma sample into a low-protein-binding microcentrifuge tube.
- Protein Precipitation: Add 200 µL of ice-cold methanol to the sample to precipitate proteins. Vortex vigorously for 30 seconds.
- Internal Standard Addition: Add 20 µL of a stable isotope-labeled internal standard solution (e.g., d4-CML, 13C6-CEL, d3-MG-H1) to correct for analyte loss and matrix effects.
- Centrifugation: Centrifuge at 14,000 x g for 15 minutes at 4°C to pellet the precipitated proteins.
- Supernatant Collection: Carefully transfer the supernatant to a new HPLC vial. The supernatant contains the glycation free adducts.
II. Liquid Chromatography (LC) Conditions
- Column: Use a reverse-phase C18 column (e.g., 2.1 x 150 mm, 1.8 µm particle size).
- Mobile Phase A: 0.1% Formic acid in water.
- Mobile Phase B: 0.1% Formic acid in acetonitrile.
- Gradient Program:
- 0-2 min: 2% B
- 2-10 min: 2% B to 30% B (linear gradient)
- 10-12 min: 30% B to 95% B (linear gradient)
- 12-14 min: Hold at 95% B
- 14-15 min: 95% B to 2% B (re-equilibration)
- 15-20 min: Hold at 2% B for column re-equilibration.
- Flow Rate: 0.25 mL/min
- Column Temperature: 40°C
- Injection Volume: 5-10 µL
III. Tandem Mass Spectrometry (MS/MS) Detection
- Ion Source: Electrospray Ionization (ESI) in positive ion mode.
- Detection Mode: Multiple Reaction Monitoring (MRM).
- Key MRM Transitions (to be optimized on specific instrumentation):
- CML: m/z 205.1 -> 130.1 (quantifier), 205.1 -> 84.1 (qualifier)
- CEL: m/z 219.1 -> 130.1
- MG-H1: m/z 229.1 -> 114.1
- d4-CML (Internal Standard): m/z 209.1 -> 134.1
- Data Analysis: Quantify analytes by calculating the peak area ratio of the analyte to its corresponding internal standard and interpolating from a linear calibration curve prepared with known standards.
The workflow for this analytical protocol is summarized below:
The Scientist's Toolkit: Key Research Reagents and Materials
Successful research into protein glycation requires a suite of specialized reagents and tools. The following table details essential items for a functional glycation research laboratory.
Table 3: Essential Research Reagents and Materials for Glycation Studies
| Reagent / Material | Function / Application | Key Considerations |
|---|---|---|
| Methylglyoxal (MGO) Solution (≥95% purity) | A primary reactive dicarbonyl precursor used to induce rapid glycation and dicarbonyl stress in vitro [56]. | Concentration must be carefully determined (e.g., via derivatization) and controlled, as MGO is highly reactive and cytotoxic. |
| AGE-BSA (in vitro) | Glycated Bovine Serum Albumin. Used as a standardized source of pre-formed AGEs for stimulating cells (e.g., via RAGE) in culture experiments [55]. | The extent and specific type of glycation (e.g., CML-BSA) should be characterized. Results should be confirmed with endogenously formed AGEs. |
| Anti-AGE Antibodies (e.g., anti-CML, anti-CEL) | Detection and localization of specific AGEs in tissues (immunohistochemistry) or biological fluids (ELISA, Western Blot) [57]. | Specificity is a major concern. Validation with knockout models or mass spectrometry is recommended to confirm antibody specificity. |
| Soluble RAGE (sRAGE) | The extracellular ligand-binding domain of RAGE. Used as a decoy receptor to inhibit AGE-RAGE signaling in vitro and in vivo [55]. | Serves as a key tool to establish the mechanistic involvement of RAGE in a observed biological effect. |
| Glyoxalase 1 (Glo1) Inhibitor (e.g., S-p-bromobenzylglutathione cyclopentyl diester) | A specific inhibitor of the primary enzyme responsible for MGO detoxification. Used to induce a state of dicarbonyl stress in cellular models [56]. | Confirms the role of endogenous MGO in a pathway. Cytotoxicity from resultant MGO accumulation must be monitored. |
| Stable Isotope-Labeled Internal Standards (e.g., d4-CML, 13C6-CEL) | Essential for accurate and precise quantification of AGEs using LC-MS/MS. Corrects for losses during sample preparation and ion suppression/enhancement in the mass spectrometer [56]. | Critical for achieving research-grade quantitative data. Should be added to the sample at the earliest possible step in preparation. |
The study of protein glycation provides a profound example of how fundamental chemical principles, first observed in food, can illuminate critical pathophysiological processes in human health and disease. The Maillard reaction, re-contextualized in vivo as glycation, is now established as a major contributor to the functional decline of tissues during aging and the development of debilitating complications in diabetes.
Future research in this field is moving in several promising directions. There is a growing emphasis on moving beyond correlative studies to establish direct causative links, particularly through the targeted manipulation of specific dicarbonyl pathways in vivo. The development of more potent and specific RAGE antagonists and glyoxalase inducers represents a vibrant area of therapeutic discovery [55] [56]. Furthermore, the integration of glycation biomarker data with other omics datasets using advanced computational approaches and machine learning holds the potential to refine risk prediction for diabetic complications and age-related diseases, ultimately paving the way for a more personalized medicine approach [56]. As our molecular understanding of glycation deepens, so too does the potential to develop effective interventions that disrupt this damaging process, promoting healthier aging and mitigating the burden of chronic metabolic disease.
In the realm of drug development, particularly for biopharmaceuticals comprising therapeutic proteins and peptides, maintaining molecular stability throughout manufacturing, storage, and shelf-life presents a formidable challenge. Among the various chemical degradation pathways, the Maillard reaction—a non-enzymatic browning reaction between carbonyl groups and amino groups—poses a significant risk to protein stability and efficacy [39]. First discovered in 1912 by Louis-Camille Maillard in food systems, this complex reaction network has profound implications for pharmaceutical formulations where proteins coexist with reducing sugars, a common scenario in biopharmaceutical products [11] [39].
The Maillard reaction is not merely a theoretical concern; it represents a primary degradation pathway for protein and polypeptide drugs when formulated with common reducing excipients such as lactose, glucose, or maltose [59]. This reaction can occur across various dosage forms, including solid formulations and liquid injections, potentially leading to color changes, reduced bioavailability, and formation of compounds with toxicological concerns [59]. For drug development professionals, understanding and mitigating this reaction is paramount for ensuring product safety, efficacy, and stability throughout the intended shelf-life.
The Maillard Reaction Mechanism and Its Implications for Therapeutic Proteins
Fundamental Reaction Stages
The Maillard reaction encompasses a complex network of chemical transformations that progresses through three characteristic stages [11] [2]:
Initial Stage: A carbonyl group from a reducing sugar condenses with a nucleophilic amino group (typically from lysine residues or terminal amines) to form an unstable Schiff base (aldimine), which spontaneously rearranges to form the more stable Amadori rearrangement product (ketoamine) [11] [39]. This stage is reversible, and no browning is apparent.
Intermediate Stage: The Amadori products undergo diverse chemical transformations including dehydration, fragmentation, and rearrangement. Key reactions include sugar fragmentation producing reactive α-dicarbonyl compounds and Strecker degradation of amino acids, yielding Strecker aldehydes and aminoketones [11] [39]. This stage generates numerous volatile flavor compounds and reactive intermediates.
Final Stage: Condensation and polymerization of intermediates occur, leading to the formation of heterogeneous, brown-colored, nitrogenous polymers known as melanoidins or advanced glycation end-products (AGEs) [11] [39]. These complex high-molecular-weight compounds are responsible for the characteristic browning.
The following diagram illustrates the key stages and pathways of the Maillard reaction in pharmaceutical systems:
Risk Factors in Pharmaceutical Formulations
Multiple factors influence the kinetics and extent of the Maillard reaction in drug formulations, which development scientists must carefully control:
Temperature and Time: Elevated temperatures during manufacturing (spray drying, lyophilization) and accelerated storage conditions significantly increase reaction rates [11] [2]. The Arrhenius relationship generally applies, with rate doubling for every 10°C temperature increase.
Water Activity: Maillard reaction proceeds optimally at intermediate water activities (a~w~ ≈ 0.5-0.8), common in solid dosage forms and lyophilized products, where molecular mobility is sufficient but dilution effects are minimal [11] [2].
pH Dependence: Reaction kinetics are highly pH-dependent, with alkaline conditions (pH >7) accelerating the reaction by increasing the concentration of unprotonated, nucleophilic amino groups [11] [2]. The pKa of amino groups dictates this relationship.
Reactant Chemistry: The specific reducing sugar and amino acid composition significantly impact reactivity. Lysine residues, with their primary ε-amino groups (pKa ≈ 10), are particularly susceptible, while sugars with open-chain carbonyl forms (e.g., lactose, maltose) show higher reactivity than non-reducing sugars [11] [6].
Analytical Methodologies for Monitoring Maillard Reaction in Pharmaceutical Systems
Comprehensive Analytical Techniques
Monitoring Maillard reaction progression and characterizing its products requires orthogonal analytical approaches capable of detecting various reaction intermediates and endpoints. The following table summarizes key methodologies employed in pharmaceutical development:
Table 1: Analytical Methods for Monitoring Maillard Reaction in Pharmaceutical Formulations
| Method Category | Specific Techniques | Application in Maillard Reaction Monitoring | Key Measurable Parameters |
|---|---|---|---|
| Spectroscopic Methods | Fourier-Transform Infrared (FTIR) Spectroscopy [13] [60] | Structural elucidation of intermediates; real-time monitoring with HPLC coupling | Functional group changes (carbonyls, imines); molecular fingerprints |
| UV-Vis Spectroscopy [13] | Browning intensity measurement | Absorbance at 294 nm (intermediates) and 420 nm (advanced products) [6] | |
| Fluorescence Spectroscopy [13] | Detection of fluorescent AGEs | Specific fluorescence emissions (λ~ex~=370 nm, λ~em~=440 nm) | |
| Separation-Based Methods | HPLC-MS/MS [13] | Quantification of specific MRPs and protein adducts | Amadori products, furosine, carboxymethyllysine (CML) |
| GC-MS/MS [13] | Analysis of volatile MRPs | Strecker aldehydes, pyrazines, furans | |
| Capillary Electrophoresis (CE) [13] | Monitoring protein glycation | Charge variants due to lysine modification | |
| Advanced Characterization | NMR Spectroscopy [13] | Structural elucidation of MRPs | Molecular structure confirmation |
| ELISA [13] [1] | Specific AGE detection | CML and other immunogenic AGEs | |
| FT-ICR-MS [6] | Non-targeted comprehensive profiling | Molecular formulae of thousands of MRPs |
Experimental Protocol: HPLC-FTIR for Real-Time Monitoring of Maillard Reaction
The combination of high-performance liquid chromatography with Fourier transform infrared spectroscopy (HPLC-FTIR) provides a powerful approach for real-time separation and structural characterization of Maillard reaction intermediates [60]. Below is a detailed methodology for implementing this technique:
Objective: To separate and structurally characterize Maillard reaction intermediates in real-time using coupled HPLC-FTIR methodology.
Materials and Reagents:
- Model System: L-Asparagine monohydrate (>99%) and fructose (>99.5%) in equimolar ratios (0.2 M) [60]
- Solvent/Buffer: Phosphate buffer (0.1 M, pH 8.0) [60]
- HPLC System: Shimadzu Prominence 20A HPLC system with quaternary pump, degasser, temperature-controlled column oven, and UV detector [60]
- Column: Zorbax SB-Aq analytical column (4.6 × 250 mm, 5 μm particle size) [60]
- FTIR System: Bruker Tensor 27 FTIR spectrometer with TGS detector and horizontal ATR accessory with Germanium flow-through cell [60]
- Mobile Phase: Water, isocratic, flow rate 0.5 ml/min at room temperature [60]
- Software: OPUS 7.0/IR/3D and OPUS CHROM for data acquisition and processing [60]
Experimental Procedure:
Sample Preparation:
- Prepare equimolar solutions (0.2 M) of fructose and asparagine in phosphate buffer (0.1 M, pH 8.0)
- Heat samples (10 ml) in closed screw-capped tubes at elevated temperatures (100, 120, 140°C) for predetermined time intervals (30, 60, 90, 120 min) [60]
- Immediately cool samples on ice after heating and store at -20°C until analysis
Instrumental Setup and Analysis:
- Configure HPLC system with isocratic mobile phase (water) at 0.5 ml/min flow rate
- Connect HPLC effluent directly to FTIR flow cell via appropriate tubing
- Set UV detection at 200 nm for monitoring MRPs and acrylamide [60]
- Collect FTIR spectra in 900-1800 cm⁻¹ range with 8 cm⁻¹ resolution and 16 co-added scans
- Achieve time resolution of 8 spectra per minute with scanner velocity of 20 kHz
- Collect background spectrum with water flowing through ATR cell before sample measurements
Data Processing and Interpretation:
- Extract spectra from 3D data set at chromatographic peak maxima
- Identify characteristic IR bands: Schiff base (C=N stretch ~1640 cm⁻¹), Amadori products, and decarboxylated intermediates
- Correlate HPLC retention times with structural information from FTIR
- Monitor time-dependent evolution of reactants and products
This protocol enables the identification of key intermediates in the asparagine-fructose Maillard pathway, including the Schiff base, Amadori products, and subsequent intermediates leading to acrylamide formation [60].
The experimental workflow for this coupled analytical approach is visualized below:
The Scientist's Toolkit: Essential Research Reagent Solutions
Successful investigation and mitigation of Maillard reaction in pharmaceutical formulations requires carefully selected reagents and reference standards. The following table outlines essential research tools:
Table 2: Research Reagent Solutions for Maillard Reaction Studies
| Reagent Category | Specific Examples | Pharmaceutical Function | Considerations for Use |
|---|---|---|---|
| Stabilizing Excipients | Cyclodextrins (α-CD, β-CD, γ-CD, HP-β-CD, SBE-β-CD) [59] | Inhibit Maillard reaction via inclusion complex formation with amino groups | Biocompatibility; formulation compatibility; inclusion efficiency |
| Alternative Sugars | Trehalose, Sucrose (non-reducing) [61] [39] | Stabilizers with reduced glycation potential | Sucrose may hydrolyze to glucose/fructose at low pH [39] |
| Amino Acid Models | Lysine, Arginine, Asparagine [6] [60] | Model nucleophiles for reactivity studies | Lysine most reactive; asparagine linked to acrylamide formation [6] |
| Reference Standards | N-ε-carboxymethyllysine (CML) [1] | AGE quantification reference | ELISA and LC-MS/MS calibration |
| Furosine [1] | Amadori product quantification | Acid hydrolysis product of lactulosyl-lysine | |
| 5-Hydroxymethylfurfural (HMF) [1] | Sugar degradation marker | Also formed in caramelization | |
| Analytical Reagents | Deuterated internal standards [1] | MS quantification accuracy | Stable isotope-labeled MRPs for precise quantification |
Strategic Mitigation Approaches in Formulation Development
Excipient Selection and Molecular Encapsulation
The strategic selection of excipients represents a primary approach for mitigating Maillard reaction in protein formulations:
Cyclodextrin Complexation: Cyclodextrins can effectively inhibit Maillard reaction by forming inclusion complexes with amino group-containing drugs. Studies demonstrate that α-cyclodextrin forms stable complexes with lysine hydrochloride, significantly reducing Maillard reaction with lactose through hydrophobic interactions, hydrogen bonding, and van der Waals forces [59]. The steric hindrance provided by cyclodextrin encapsulation physically separates reactive amino and carbonyl groups.
Sugar Selection and Replacement: Where possible, replacing reducing sugars with non-reducing alternatives (trehalose, sucrose) minimizes glycation risk. However, the potential for hydrolysis of disaccharides to reducing monosaccharides must be evaluated under formulation pH conditions [61] [39]. Smaller sugar alcohols may provide stabilization without carbonyl reactivity.
Processing and Storage Optimization
Controlled Temperature and Time Profiles: Implementing moderate temperature conditions during manufacturing processes such as spray drying, lyophilization, and spray congealing minimizes thermal exposure while achieving target product characteristics [11] [2].
Moisture Content Management: For solid dosage forms, maintaining low moisture content (a~w~ <0.3) reduces molecular mobility necessary for Maillard reaction propagation, while excessively dry conditions may promote other degradation pathways [11] [2].
pH Optimization: Formulating at mildly acidic pH (pH 4-6) where feasible reduces the concentration of unprotonated amino groups, thereby slowing the initial glycation step while considering protein solubility and stability requirements [11] [2].
The Maillard reaction presents a significant challenge in developing stable protein therapeutics, particularly as biopharmaceuticals continue to comprise an expanding proportion of the pharmaceutical pipeline. Understanding the reaction mechanism, monitoring its progression with sophisticated analytical methodologies, and implementing strategic formulation approaches are essential competencies for drug development scientists.
Future directions in managing Maillard reaction liabilities include the development of site-specific protein engineering to modify reactive lysine residues, advanced computational modeling to predict glycation hotspots, and novel excipient systems that provide stabilization without participating in deleterious side reactions. Furthermore, as regulatory scrutiny of protein degradation products intensifies, comprehensive characterization and control of Maillard reaction pathways will become increasingly critical to successful biopharmaceutical development.
By integrating fundamental chemistry knowledge with practical formulation strategies, drug development professionals can effectively mitigate Maillard reaction risks, ensuring the delivery of safe, stable, and efficacious protein therapeutics to patients.
The Maillard reaction (MR), a complex network of non-enzymatic reactions between reducing sugars and amino compounds, is a cornerstone of food chemistry and a significant contributor to the molecular aging of proteins in vivo. This reaction generates a vast spectrum of Maillard reaction products (MRPs) with diverse chemical structures and biological activities. The duality of MRPs—acting as potent antioxidants under certain conditions while exhibiting pro-oxidant or even toxicological effects under others—presents a critical area of investigation for food scientists, nutritionists, and toxicologists. Framed within the broader context of non-enzymatic browning research, this assessment delves into the mechanisms governing this dual functionality. Understanding the precise chemical nature and contextual behavior of MRPs is paramount for optimizing food processing to maximize health benefits and minimize potential risks, thereby informing both public health and therapeutic development.
The Maillard Reaction: Mechanism and Stages
The Maillard reaction is not a single entity but a complex cascade of reactions, traditionally divided into three overlapping stages: initial, intermediate, and final [11] [28] [2]. The process begins with a nucleophilic addition, where a free amino group from an amino acid, peptide, or protein condenses with the carbonyl group of a reducing sugar (e.g., glucose, fructose) to form an unstable Schiff base [11] [2]. This compound rapidly rearranges into a more stable Amadori or Heyns product, marking the end of the initial stage [11].
Subsequently, the reaction progresses to the intermediate stage, where the Amadori products undergo diverse degradation pathways—including dehydration, enolization, fragmentation, and Strecker degradation—influenced heavily by the system's pH [11] [2]. This stage yields a plethora of highly reactive carbonyl compounds, including α-dicarbonyls (e.g., glyoxal, methylglyoxal), furans, and pyrazines, which are pivotal precursors for both flavor compounds and advanced glycation end-products (AGEs) [11].
The final stage involves the polymerization of these reactive intermediates, leading to the formation of high molecular weight, brown nitrogenous polymers known as melanoidins [11] [28]. The specific pathway and the nature of the end-products are critically dependent on reaction conditions such as temperature, time, water activity, pH, and the types of reacting sugar and amino acid [11] [2].
MRPs as Antioxidants: Mechanisms and Evidence
A significant body of research highlights the potent antioxidant capabilities of MRPs, which function through several key mechanisms.
Primary Antioxidant Mechanisms
- Free Radical Scavenging: MRPs, particularly melanoidins and intermediate compounds like reductones, can directly donate hydrogen atoms to free radicals, thereby terminating chain-propagation reactions [62]. Studies using model systems have demonstrated this activity through various chemical assays.
- Metal Chelation: MRPs possess a strong ability to chelate pro-oxidant metal ions, such as iron (Fe²⁺) and copper (Cu²⁺) [63] [62]. By sequestering these metals, MRPs prevent them from catalyzing the Fenton reaction, a primary source of highly destructive hydroxyl radicals (HO•) [64].
- Destruction of Peroxides: Some MRPs can interact with and destroy hydrogen peroxide and lipid peroxides, preventing the formation of secondary reactive oxygen species [62].
Experimental Evidence and Model Systems
Evidence for the antioxidant activity of MRPs is robust across both chemical and cellular models. For instance, MRPs derived from a histidine-glucose model system exhibited significant peroxyl radical scavenging activity as measured by the Oxygen Radical Absorbance Capacity (ORAC) assay [63]. The antioxidant activity was found to increase with heating time and temperature, correlating with the formation of melanoidins [63].
In more complex biological models, pre-treating differentiated Caco-2 intestinal cells with MRPs from a fructose-glycine model system protected against ethanol-induced loss of trans-epithelial electrical resistance (TEER) and reduced disruption of tight-junction proteins, indicating a protective, antioxidant-like effect on the intestinal barrier [65]. Notably, this protective activity was attributed to a low molecular weight (<1 kDa) fraction, underscoring that bioactivity is not exclusive to high molecular weight melanoidins [65].
Table 1: Summary of Key Antioxidant Assays for MRP Evaluation
| Assay Name | Target Radical/Species | Mechanism of Action Measured | Example Application on MRPs |
|---|---|---|---|
| ORAC (Oxygen Radical Absorbance Capacity) [65] [63] | Peroxyl radicals (ROO•) | Hydrogen atom transfer (HAT); measures the inhibition of peroxyl radical-induced fluorescein decay. | MRPs from glucose-histidine showed increased peroxyl radical scavenging with heating time [63]. |
| DPPH (2,2-Diphenyl-1-picrylhydrazyl) [65] | Stable DPPH radical | Single electron transfer (SET) or HAT; measures the decrease in DPPH absorption at 517nm. | Used to assess free-radical scavenging capacity of MRPs from various sugar-amino acid models [65]. |
| TEAC (Trolox Equivalent Antioxidant Capacity) [65] | ABTS⁺• cation radical | SET; measures the ability to scavenge the pre-formed ABTS radical cation. | Employed to compare radical scavenging of MRPs against a Trolox standard [65]. |
| Metal Chelation Assay | Fe²⁺ or Cu²⁺ ions | Chelation; measures the reduction in the formation of colored metal-chelate complexes. | MRPs from heated histidine and glucose demonstrated copper ion binding ability [63]. |
The Pro-Oxidant and Toxicological Face of MRPs
Despite their beneficial antioxidant properties, certain MRPs can promote oxidative stress and exhibit direct toxicological effects, primarily through two interconnected pathways: the induction of oxidative distress and the formation of specific harmful compounds.
Induction of Cellular Oxidative Stress
A key mechanism underlying the pro-oxidant and inflammatory effects of MRPs, particularly Advanced Glycation End-products (AGEs), is the activation of the Receptor for AGEs (RAGE) [66]. RAGE is a multi-ligand cell surface receptor expressed on various immune and other cell types. The binding of AGEs to RAGE activates the transcription factor NF-κB, leading to the increased expression of pro-inflammatory cytokines and a sustained generation of reactive oxygen species (ROS), thereby creating a state of chronic oxidative stress and inflammation [66]. This pathway is implicated in the pathogenesis of diabetes, cardiovascular diseases, and neurodegenerative disorders [66].
Table 2: Harmful Compounds Formed via the Maillard Reaction
| Compound | Precursors | Toxicological Concern |
|---|---|---|
| Acrylamide [11] [30] | Asparagine and reducing sugars (e.g., in potatoes, cereals). | Classified as a probable human carcinogen; forms during high-temperature processing (e.g., frying, baking) [11] [30]. |
| Heterocyclic Amines (HCAs) [30] | Amino acids and creatine (e.g., in cooked muscle meats). | Associated with an increased risk of various cancers in epidemiological studies [30]. |
| Advanced Glycation End Products (AGEs) (e.g., CML, CEL) [30] [66] | Proteins and reducing sugars. | Implicated in promoting oxidative stress and inflammation via RAGE activation, contributing to diabetes complications and cardiovascular disease [66]. |
Context-Dependent Duality
The transition of MRPs from antioxidants to pro-oxidants is not absolute but highly context-dependent. Factors such as the stage of the reaction, the specific molecular weight fraction, and the cellular or chemical environment are critical. For example, while many studies report antioxidant activity in MRPs, some early-stage intermediates can exhibit pro-oxidant behavior under specific conditions, such as in the presence of redox-active metal ions [63]. Furthermore, as evidenced in a soy protein-glucose model, prolonged heating time led to increased formation of AGEs (e.g., CML), which was positively correlated with binding to the RAGE receptor and the subsequent stimulation of pro-inflammatory cytokines (IL-1β, IL-8, TNF-α) in monocytes [66]. This demonstrates that the same reaction that generates antioxidant melanoidins can also produce pro-inflammatory compounds, with the balance shifting over time and with changing reaction conditions.
Essential Methodologies for MRP Analysis
A rigorous assessment of the dual role of MRPs requires a combination of analytical techniques to characterize the reaction products and evaluate their biological activity.
Key Experimental Protocols
1. Generating MRPs in Model Systems: A common methodology involves dry or wet heating of simplified model systems. For example, a standard protocol involves mixing a reducing sugar (e.g., glucose) and an amino acid (e.g., glycine, histidine) in a 1:1 or 1:2 molar ratio, with or without a non-reactive bulking agent like Alphacel (microcrystalline cellulose) to simulate a food matrix [65] [63]. The mixture is then heated in an oven (e.g., at 150°C for 20-40 min for dry heating, or at 100°C for various durations in a screw-capped tube for aqueous heating) [65] [63]. The resulting water-soluble MRPs are recovered through centrifugation, filtration, and lyophilization.
2. Evaluating Antioxidant Capacity:
- ORAC Assay: This protocol involves adding the MRP sample to a solution of fluorescein. The peroxyl radical generator AAPH (2,2'-azobis(2-amidinopropane) dihydrochloride) is added, and the decay of fluorescein fluorescence is monitored over time. The area under the fluorescence decay curve is compared to that of a Trolox standard to calculate the ORAC value [65] [63].
- DPPH Assay: A methanolic solution of the DPPH• radical is mixed with the MRP sample. The reaction is incubated in the dark, and the reduction of the DPPH radical is measured by the decrease in absorbance at 517 nm [65].
3. Assessing Bio-functional Activity in Cell Models:
- Transepithelial Electrical Resistance (TEER): Differentiated Caco-2 cells grown on permeable inserts are pre-treated with MRPs and then exposed to an insult (e.g., 7.5% ethanol). TEER, a measure of intestinal barrier integrity, is measured over time using a volt-ohmmeter [65].
- Inflammatory Marker Analysis: Immune cells like peripheral blood monocytes are stimulated with glycated proteins (e.g., soy protein-glucose MRPs). The expression of pro-inflammatory cytokine genes (IL-1β, IL-8, TNF-α) is quantified using methods like RT-qPCR, and the binding to the RAGE receptor can be assessed via competitive binding assays [66].
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Reagents for MRP and Oxidative Stress Research
| Reagent / Assay Kit | Function / Application | Research Context |
|---|---|---|
| AAPH (2,2'-azobis(2-amidinopropane) dihydrochloride) | Peroxyl radical generator for the ORAC assay. | Essential for standardized evaluation of the peroxyl radical scavenging capacity of MRPs [65] [63]. |
| DPPH (2,2-Diphenyl-1-picrylhydrazyl) | Stable free radical for the DPPH antioxidant assay. | Used for rapid, high-throughput screening of MRPs' free radical scavenging activity [65]. |
| Caco-2 Cell Line (HTB-37) | Model of human intestinal epithelium. | Used to study the protective effects of MRPs on gut barrier function (via TEER measurements) and nutrient transport [65]. |
| Primary Antibodies (Claudin-4, Occludin, ZO-1) | Target tight-junction proteins for immunofluorescence or Western blot. | Applied to visualize and quantify the integrity of the Caco-2 cell barrier after MRP treatment and insult [65]. |
| RAGE Binding / Inhibition Assay Kit | Measures the interaction between MRPs/AGEs and the RAGE receptor. | Critical for investigating the pro-inflammatory potential of dietary AGEs and their role in activating inflammatory signaling pathways [66]. |
The Maillard reaction yields a complex and dynamic mixture of products whose biological impact is fundamentally dualistic. MRPs can function as effective antioxidants, scavenging free radicals, chelating metals, and protecting cellular systems from oxidative damage. Conversely, they can also act as pro-oxidants by generating reactive carbonyl species, forming covalent adducts with proteins, and triggering RAGE-mediated inflammatory pathways that perpetuate oxidative stress. This duality is not a contradiction but a reflection of the chemical heterogeneity of MRPs and the sensitivity of the Maillard reaction to its environment. The net effect—beneficial or detrimental—is dictated by precise reaction conditions, including the nature of the precursors, thermal load, and the stage of the reaction, as well as the biological context of exposure. Future research must continue to elucidate the intricate structure-activity relationships of specific MRPs, moving beyond bulk mixtures to defined compounds. This refined understanding is crucial for harnessing the Maillard reaction's potential to enhance food quality and safety, while mitigating its role in diet-related chronic diseases.
Controlling the Reaction: Strategies to Modulate Maillard Pathways and Outcomes
Non-enzymatic browning (NEB), particularly the Maillard reaction, is a complex network of chemical reactions that profoundly influences the quality, safety, and nutritional characteristics of processed foods and pharmaceuticals. This reaction, first described by Louis Camille Maillard in 1912, occurs between nucleophilic amino groups (e.g., from amino acids, peptides, or proteins) and carbonyl groups (primarily from reducing sugars) [11]. The Maillard reaction is not a single pathway but a cascade of reactions leading to a wide array of products, including melanoidins (brown pigments), aroma compounds, and advanced glycation end-products (AGEs) [11] [1]. Alongside the Maillard reaction, caramelization (thermal degradation of sugars) also contributes significantly to non-enzymatic browning [1].
The control and optimization of these reactions in industrial applications—ranging from food flavor development to drug formulation stability—require a deep understanding of the critical parameters that govern their kinetics and pathways. The pH value of the medium is a key parameter controlling the kinetics of the Maillard reaction, as it directly influences the concentration of nucleophilic groups available for the initial reaction step [11]. Other specific conditions of the reaction medium such as temperature, reaction time (or residence time in a process), and water activity also significantly influence the Maillard reaction's progression and the nature of the products formed [11] [67].
This technical guide provides an in-depth analysis of how these four critical parameters—temperature, time, pH, and water activity—influence the Maillard reaction and non-enzymatic browning processes. By synthesizing current research and experimental data, we aim to equip researchers, scientists, and drug development professionals with the knowledge to predict, control, and optimize these chemically complex reactions within the broader context of non-enzymatic browning research.
The Maillard reaction mechanism progresses through three distinct stages, each characterized by specific types of chemical transformations and intermediate products [11] [1].
Initial Stage
The reaction begins with a condensation between the carbonyl group of a reducing sugar and a free amino group (typically from an amino acid or protein), forming an unstable Schiff base. This Schiff base then undergoes rearrangement to form a more stable Amadori rearrangement product (or Heyns product in the case of ketoses) [11] [1]. This stage is reversible, and no browning is apparent at this point [1].
Intermediate Stage
The Amadori or Heyns products undergo further transformations through various pathways depending on pH conditions. At pH ≤ 7, these products mainly undergo 1,2-enolization, forming furfurals (from pentoses) or hydroxymethylfurfural (HMF) (from hexoses) [1]. At higher pH (>7), 2,3-enolization is favored, producing reductones and fission products [1]. This stage also includes the Strecker degradation, where α-dicarbonyl compounds react with amino acids to produce Strecker aldehydes (contributing to aroma) and aminoketones [11] [1].
Final Stage
The final stage involves condensation, polymerization, and cyclization reactions of the various intermediates formed in the previous stages. This leads to the formation of heterocyclic nitrogenous polymers known as melanoidins, which are responsible for the characteristic brown color [11] [1]. It is at this stage that potentially harmful compounds such as acrylamide and advanced glycation end-products (AGEs) can form [11].
The following diagram illustrates the interconnected nature of the Maillard reaction pathway and the points at which critical parameters exert their influence:
Figure 1: Maillard Reaction Pathway and Parameter Influence. This diagram illustrates the three-stage Maillard reaction mechanism and shows how critical parameters (temperature, time, pH, and water activity) influence key steps in the pathway.
Critical Parameter Analysis
Temperature
Temperature is arguably the most significant extrinsic factor controlling the rate of non-enzymatic browning reactions. The relationship between temperature and reaction rate generally follows the Arrhenius equation, which describes the exponential increase in reaction rate with increasing temperature [68].
Table 1: Temperature Effects on Maillard Reaction Parameters
| Temperature Range | Reaction Rate | Product Formation | Industrial Applications |
|---|---|---|---|
| 20-50°C | Very slow | Minimal browning; primarily early stage intermediates | Storage conditions to prevent quality deterioration [67] |
| 60-100°C | Moderate | Noticeable browning; flavor compound development | Pasteurization, blanching, liquid food processing [68] |
| 110-150°C | Rapid | Extensive browning; diverse flavor and aroma compounds | Baking, roasting, frying, extrusion cooking [69] |
| >150°C | Very rapid | Potentially excessive browning; harmful compound formation | Grilling, high-temperature roasting [11] |
The activation energy (Ea) for Maillard reaction and browning formation varies significantly depending on the reaction system and conditions. For instance, in ascorbic acid-glycine model systems, the Ea for the formation of un-colored intermediate products (UIPs) was approximately 53.76 kJ/mol at pH 4.5, while the Ea for browning products (BPs) formation was approximately 94.06 kJ/mol under the same conditions [69]. This indicates that the formation of BPs has a higher temperature dependence than the formation of UIPs, particularly in acidic environments.
In practical applications, temperature control is crucial for directing the Maillard reaction toward desirable outcomes. For example, in the production of fish soup powder, storage at 20°C resulted in a shelf life of 155 days before reaching a critical peroxide value (indicating oxidation), while storage at 50°C reduced the shelf life to just 108 days [67].
Time
The duration of thermal exposure directly influences the extent and progression of the Maillard reaction through its various stages. Time and temperature often exhibit interactive effects, with higher temperatures requiring shorter times to achieve similar levels of browning [68] [69].
The kinetics of browning development often follows pseudo-zero-order kinetics in many food systems, particularly in intermediate-moisture and low-moisture products [67] [68]. However, in liquid model systems, the browning development may exhibit more complex kinetics. Research on glycine-glucose systems has shown that the conversion of absorbance values to "% unaccomplished transmittance" often reveals sigmoidal curves with two points of abrupt slope changes, corresponding to two distinct browning phases [68].
The time parameter interacts strongly with other factors. For instance, the optimal heating time varies significantly with pH. In a study on ascorbic acid-glycine systems at 150°C, the absorbance of browning products (A420) reached approximately 17.5 after 150 minutes at pH 4.5, while at pH 9.5, similar browning intensity was achieved in just 60 minutes [69].
In industrial processes, time-temperature combinations are carefully optimized to achieve desired quality attributes while minimizing the formation of undesirable compounds. For example, in the flue-curing of tobacco, specific time-temperature protocols are employed during the yellowing and color-fixing stages to control the degree of browning, which directly impacts product quality and value [70].
pH
The pH of the reaction medium profoundly influences both the kinetics and pathways of the Maillard reaction by affecting the ionization states of the reactants, particularly the amino groups [11] [71].
Table 2: pH Influence on Maillard Reaction Pathways and Products
| pH Range | Reaction Characteristics | Dominant Products | Reaction Rate |
|---|---|---|---|
| Acidic (pH < 5) | Favors 1,2-enolization; protonation of amino groups reduces nucleophilicity | Furfural, HMF; less browning [1] [71] | Slow [71] |
| Neutral (pH 6-7) | Moderate nucleophile availability; balanced pathway | Mixed products; early browning [11] | Moderate |
| Alkaline (pH 8-10) | Deprotonated amino groups enhance nucleophilicity; favors 2,3-enolization | Reductones, fission products; intense browning [1] [71] | Fast [71] |
The effect of pH on browning intensity can be dramatic. Ashoor and Zent (1984) reported that no browning was observed in any amino acid-glucose mixtures below pH 6.0, with maximum browning occurring at pH 10.0 [71]. Similarly, in γ-irradiation processing of sugar-glycine solutions, browning increased significantly at alkaline pH values, particularly at pH 10.0 [71].
pH also affects the activation energy of browning reactions. Research has shown that the lower the pH, the higher the activation energy for the formation of brown polymers [71]. Furthermore, pH significantly influences the formation of specific classes of aroma compounds. At higher pH values, there is enhanced formation of furanones and pyrazines, while lower pH favors the formation of furans [71].
The choice of buffering agents is an important consideration in experimental systems, as different buffer types can influence the Maillard reaction rate and pathway through specific ion effects [71].
Water Activity (a~w~)
Water activity (a~w~) represents the amount of available water in a system and profoundly influences the Maillard reaction by affecting reactant mobility, concentration, and stability of intermediates [11] [67].
The relationship between water activity and the rate of non-enzymatic browning typically follows a bell-shaped curve. At very low water activities (a~w~ < 0.3), the reaction rate is minimal due to restricted molecular mobility. As water activity increases to the range of 0.6-0.8, the reaction rate reaches its maximum, as water provides sufficient mobility for reactants while maintaining optimal concentration through solute dissolution. Beyond a~w~ ~ 0.8, the reaction rate decreases due to dilution of reactants [67].
In dehydrated fish powder, the relationship between relative humidity (which correlates with water activity) and oxidative stability showed a minimum rate at a water activity range between 0.3 and 0.5, while the rate of non-enzymatic browning was less affected by relative humidity in this range [67]. This highlights the differential effect of water activity on various degradation pathways in low-moisture systems.
Water activity also interacts with temperature in controlling reaction rates. In low-moisture systems, the effect of temperature becomes more pronounced at intermediate water activities where molecular mobility is sufficient to allow reactions to proceed. Additionally, water activity influences the glass transition temperature (T~g~) of food matrices, which in turn affects molecular mobility and reaction rates [67].
Interparameter Relationships and Modeling
The critical parameters influencing non-enzymatic browning do not operate in isolation but exhibit complex interactions that can be synergistic, antagonistic, or compensatory. Understanding these interparameter relationships is essential for predictive modeling and process optimization.
Parameter Interactions
Temperature-pH interactions are particularly significant. At higher temperatures, the effect of pH on reaction pathways becomes more pronounced. For instance, the differential formation of furans versus furanones at different pH values is amplified at elevated temperatures [71] [69].
Temperature-water activity interactions follow complex patterns. In low-moisture systems, the temperature dependence of the Maillard reaction increases with increasing water activity up to an optimum point, after which dilution effects diminish this dependence [67].
Time-pH interactions manifest in the changing dominance of reaction pathways over extended periods. Alkaline conditions typically accelerate the initial stages of the Maillard reaction but may lead to reaction stagnation or decline due to reactant depletion or inhibitory product formation [68] [69].
The following diagram illustrates the complex interplay between these critical parameters:
Figure 2: Interparameter Relationships in Non-Enzymatic Browning. This diagram illustrates the complex interactions between the four critical parameters and their collective impact on Maillard reaction kinetics and pathways.
Mathematical Modeling Approaches
Multiple mathematical approaches have been developed to describe and predict the combined effects of critical parameters on non-enzymatic browning:
Kinetic models typically employ zero-order, first-order, or more complex sigmoidal functions (such as the modified Gompertz model) to describe browning development over time [68]. The temperature dependence is often incorporated using the Arrhenius equation [68].
Multiple-factor modeling approaches have been developed to express non-enzymatic browning development as a function of time, temperature, pH, and substrate concentration ratio simultaneously. These models often combine modified Gompertz and Arrhenius equations with interaction polynomial models to account for parameter interdependencies [68].
Composite kinetic models have been successfully applied to describe quality changes in complex food systems during storage. For example, such models have effectively described the effects of environmental, iso-thermal, and iso-relative humidity conditions on quality changes in packaged fish powder [67].
Experimental Protocols and Methodologies
Model System Preparation
Aqueous model systems provide a controlled environment for studying non-enzymatic browning. A typical protocol involves [68] [69]:
Prepare solutions of reducing sugars (e.g., glucose, ribose, fructose) and amino acids (e.g., glycine, lysine) in various molar ratios (commonly 0.1-2.0 M) using appropriate buffer systems.
Adjust pH using appropriate buffer systems (e.g., phosphate buffers for neutral pH, carbonate-bicarbonate for alkaline pH) across the desired range (typically pH 4.0-10.0) [71] [69].
Transfer solutions to sealed reaction vessels (e.g., glass vials, ampoules) to prevent moisture loss during heating.
Heat samples at controlled temperatures (ranging from 40°C to 150°C depending on the study objectives) for predetermined time periods [68] [69].
Terminate reactions by rapid cooling (ice-water bath) at specific time intervals.
Analytical Methods for Monitoring Browning
Spectrophotometric methods are widely used for quantifying browning intensity:
- Absorbance at 294 nm: Measures the formation of uncolored intermediate products (UIPs) [6] [69].
- Absorbance at 420 nm: Measures the formation of brown pigments (BPs) in the final stage of the Maillard reaction [71] [68] [69].
- Spectrophotometric indices: For samples with inherent color, appropriate dilution factors (25-, 50-, or 100-fold) are applied, with final absorbance values calculated by multiplying actual absorbance by the dilution factor [69].
Colorimetric measurements in the CIE Lab* color space are commonly applied to monitor visual browning in solid and semi-solid products [1] [72]. The browning index (BI) or total color difference (ΔE) can be calculated using standardized equations [72] [68].
Chromatographic techniques provide detailed analysis of specific reaction products:
- High-Performance Liquid Chromatography (HPLC): Quantifies specific intermediates and products, including hydroxymethylfurfural (HMF), furosine, and amino acid substrates [1] [69].
- Liquid Chromatography-Mass Spectrometry (LC-MS): Enables identification and quantification of specific MRPs, including advanced glycation end-products (AGEs) like N(ε)-carboxymethyllysine (CML) [1].
- Gas Chromatography-Mass Spectrometry (GC-MS): Ideal for identifying low-molecular-weight volatile compounds, including Strecker aldehydes, furans, and pyrazines [1] [6].
Advanced analytical techniques:
- Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR-MS): Provides ultrahigh-resolution mass analysis for comprehensive characterization of MRPs, enabling assignment of unambiguous molecular formulae to hundreds of reaction products simultaneously [6].
- Fluorescence spectroscopy: Can distinguish between different browning stages by detecting fluorescent MRPs [1].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagent Solutions for Maillard Reaction Studies
| Reagent/Material | Function/Application | Technical Considerations |
|---|---|---|
| Reducing Sugars (e.g., Glucose, Fructose, Ribose, Lactose) | Carbonyl group donors; reactivity varies with sugar structure | Ribose shows high reactivity among pentoses and hexoses; fructose and glucose have different reaction patterns [11] [6] |
| Amino Acids (e.g., Glycine, Lysine, Cysteine, Arginine) | Amino group donors; side chains influence reaction pathways | Lysine > cysteine > isoleucine ≈ glycine in reactivity order; cysteine suppresses browning but produces diverse MRPs [11] [6] |
| Buffer Systems (e.g., Phosphate, Acetate, Carbonate-Bicarbonate) | pH control; different buffers can influence reaction rates | Phosphate buffers may catalyze reactions; buffer type and concentration should be standardized [71] |
| Ascorbic Acid | Polyhydroxy compound generating carbonyls upon heating; participates in NEB | Degrades to reactive carbonyl species; contributes to browning in fruit/vegetable products [11] [69] |
| Spectrophotometer (UV-Vis) | Quantification of UIPs (294 nm) and BPs (420 nm) | Requires appropriate dilution factors for highly colored samples; cuvette path length standardization [69] |
| Analytical Standards (e.g., HMF, Furosine, CML) | Quantification of specific MRPs | Commercial ELISA kits for CML show poor specificity; LC-MS/MS methods preferred for accuracy [1] |
| Sealed Reaction Vessels (e.g., glass vials, ampoules) | Prevent moisture loss during heating | Headspace oxygen can influence oxidation pathways; inert atmosphere may be required [69] |
The critical parameters of temperature, time, pH, and water activity exert profound and interconnected influences on non-enzymatic browning reactions. Temperature accelerates reaction rates exponentially according to Arrhenius kinetics, with activation energies varying significantly between different stages of the reaction. Time determines the progression through initial, intermediate, and final stages of the Maillard reaction, with kinetics ranging from pseudo-zero-order to complex sigmoidal patterns. pH controls both reaction rate and pathway by influencing the ionization states of reactants, directing the formation of specific classes of flavor and color compounds. Water activity governs molecular mobility and reactant concentration, exhibiting optimal ranges for maximal reaction rates.
The complex interplay between these parameters necessitates sophisticated modeling approaches that can account for their synergistic and antagonistic interactions. Multiple-factor mathematical models combining kinetic, Arrhenius, and polynomial elements show promise for predicting browning development under varying conditions. From an experimental perspective, appropriate selection of model systems, analytical techniques, and reagent solutions is essential for generating reproducible and mechanistically insightful data.
For researchers and product developers, mastery of these critical parameters enables the precise control of non-enzymatic browning reactions—whether the goal is to enhance desirable attributes like flavor and color or to minimize the formation of nutritionally detrimental compounds. As analytical technologies continue to advance, particularly in the realm of high-resolution mass spectrometry, our understanding of parameter effects on the complex network of Maillard reaction pathways will continue to deepen, enabling ever more sophisticated control strategies for both food and pharmaceutical applications.
The Maillard reaction, a form of non-enzymatic browning, represents a complex network of chemical reactions between carbonyl compounds (typically reducing sugars) and amino compounds (primarily amino acids, peptides, or proteins) [2] [73]. Discovered in 1912 by Louis Camille Maillard, this reaction significantly influences food quality parameters including color, flavor, aroma, and nutritional value [2] [74]. Within the context of non-enzymatic browning research, understanding precursor chemistry is paramount for controlling reaction outcomes in both food systems and physiological environments. The specific chemical properties of the reactants—particularly the nucleophilicity of amino groups and the redox potential of sugars—directly determine reaction kinetics, pathway selection, and ultimate product distribution [2] [75] [73].
The fundamental importance of precursor chemistry extends beyond food science into biomedical research. Advanced Glycation End-products (AGEs), formed through Maillard reactions under physiological conditions, contribute to protein cross-linking, tissue aging, and various pathologies including diabetes, cardiovascular diseases, and neurodegenerative disorders [48]. Consequently, elucidating how specific amino acid side chains and sugar types dictate reactivity is crucial for developing intervention strategies against AGE-related pathologies and for optimizing Maillard reaction applications in industrial processes [2] [48].
Fundamental Chemistry of Maillard Reaction Initiation
The Maillard reaction commences with a nucleophilic addition reaction, where a Lewis base (nucleophile) attacks an electrophilic carbonyl carbon [2]. This initial step forms a tetrahedral intermediate that serves as the gateway to diverse reaction pathways [2]. The specific nature of this nucleophilic attack is profoundly influenced by precursor chemistry, particularly the pH-dependent nucleophilicity of amino groups and the carbonyl reactivity of sugars.
The reaction progresses through three main stages [2]:
- Early Stage: Condensation of carbonyl groups from reducing sugars with nucleophilic amino groups to form Schiff bases, which subsequently rearrange into more stable Amadori rearrangement products (for aldoses) or Heyns rearrangement products (for ketoses) [2] [75] [73].
- Intermediate Stage: Degradation of rearrangement products through various pathways (1,2- or 2,3-enolization) influenced by pH conditions, yielding reactive intermediates including α-dicarbonyl compounds, furfurals, and reductones [2] [29].
- Final Stage: Condensation, polymerization, and cyclization reactions forming melanoidins—brown nitrogenous polymers—along with numerous flavor and aroma compounds [2] [74] [29].
The following diagram illustrates the core mechanistic workflow of the Maillard reaction, highlighting key decision points influenced by precursor chemistry:
Amino Acid Side Chains and Their Influence on Reactivity
The structural characteristics of amino acid side chains fundamentally determine Maillard reaction kinetics and product profiles. The nucleophilic strength of the amino group, governed by its pKa value and molecular environment, dictates the initial condensation rate with carbonyl compounds [2]. Different amino group sources exhibit varying reactivity, with side chain chemistry influencing both reaction rate and flavor compound specificity.
Nucleophilic Group Classification and Reactivity
Amino acids participate in Maillard reactions primarily through their nucleophilic groups, with reactivity dependent on pH conditions that affect their protonation state [2]:
Table 1: Nucleophilic Groups in Amino Acids and Their Maillard Reaction Parameters
| Nucleophilic Group | Amino Acid Example | pKa Value | pH Optimization | Reaction Characteristics |
|---|---|---|---|---|
| Primary amino groups | Lysine | ~10.0 | pH > 8.0 | High browning intensity; produces pyrazines and alkylated pyridines |
| Secondary amino groups | Histidine | ~6.5 | pH > 5.5 | Moderate reactivity; influences cyclic N-heterocycle formation |
| Thiolate groups | Cysteine | ~9.0 | pH > 8.0 | S-Maillard pathway; generates sulfur-containing compounds (thiazoles, thiophenes) |
| Guanidine groups | Arginine | ~12.5 | pH > 11.0 | Low participation due to high pKa; minimal browning |
The concentration of reactive nucleophilic forms follows the equilibrium: Nu+H ⇌ H+ + Nü, where higher pH values favor the deprotonated, nucleophilic form (Nü) [2]. This relationship explains why Maillard reaction kinetics are highly pH-dependent, with optimal rates occurring when the pH exceeds the pKa of the participating amino group [2].
Side Chain Structure and Product Specificity
Beyond nucleophilicity, amino acid side chains direct the formation of specific flavor and aroma compounds through their chemical properties [75] [73]. Ashoor and Zent classified amino acids based on browning intensity when heated with reducing sugars, identifying lysine, glycine, tryptophan, and tyrosine as highly reactive [75]. O'Brien and Morrissey further noted lysine's exceptional reactivity due to its two available amino groups [75].
The unique reaction pathways enabled by specific side chains include:
- Sulfur-containing side chains (cysteine, methionine): Generate characteristic meaty, roasted aromas through formation of thiazoles, thiophenes, and other sulfur heterocycles [2].
- Alkyl side chains (leucine, isoleucine): Produce chocolate, nutty, or fruity aromas through Strecker degradation and subsequent aldol condensations [73].
- Aromatic side chains (phenylalanine, tyrosine): Yield floral, almond-like aromatics and contribute to melanoidin chromophore formation [75] [73].
- Basic side chains (lysine, arginine, histidine): Enhance browning rates and produce nitrogen-rich heterocyclic compounds including pyrazines and pyridines [2] [75].
Sugar Chemistry and Structural Influences on Reactivity
The carbonyl component of the Maillard reaction, typically a reducing sugar, contributes significantly to reaction kinetics and product distribution through its chemical structure, ring conformation, and reducing potential [2] [75]. Sugar reactivity determines the initial condensation rate with amino compounds and influences degradation pathways during intermediate reaction stages.
Structural Categories of Reducing Sugars
Table 2: Structural Classification of Reducing Sugars and Maillard Reactivity
| Sugar Category | Representative Sugars | Structural Features | Relative Reactivity | Characteristic Products |
|---|---|---|---|---|
| Aldohexoses | Glucose, Galactose | Aldehyde functional group | Moderate | Furans, pyranones |
| Ketohexoses | Fructose, Sorbose | Ketone functional group | Higher than aldoses | Diacetyl, hydroxyacetone |
| Aldopentoses | Ribose, Xylose | 5-carbon aldose | Very high | Furfural, malty aromas |
| Disaccharides | Lactose, Maltose | Glycosidic bonds | Low to moderate | Diverse flavor compounds |
| Amino sugars | Glucosamine | Combined amino and carbonyl | Highest | Intense browning, specialty flavors |
The open-chain form of reducing sugars, though present in minimal quantities (less than 1% for glucose in aqueous solutions), is crucial for initiating the Maillard reaction, as it provides the electrophilic carbonyl group necessary for nucleophilic attack [75]. Sugar isomerization between cyclic and open-chain forms represents a fundamental equilibrium that influences overall reaction rate [75].
Comparative Reactivity of Sugar Structures
Experimental studies directly comparing sugar reactivity demonstrate significant differences in browning potential. Research on individual Maillard reactants showed that glucosamine (possessing both carbonyl and amino functionalities) exhibited significantly higher reactivity compared to glucose, cyclohexylamine, or benzylamine when heated at 120°C for 1-3 hours [75]. This enhanced reactivity stems from glucosamine's molecular structure containing both reactive functional groups, facilitating intramolecular catalysis [75].
Among conventional sugars, pentoses (5-carbon sugars) generally demonstrate higher reactivity than hexoses (6-carbon sugars), while ketoses show greater reactivity than aldoses [2] [73]. For instance, fructose (a ketohexose) exhibits faster browning rates than glucose (an aldohexose) under equivalent conditions [2]. This reactivity hierarchy directly influences industrial applications, with high-reactivity sugars preferred for rapid flavor development and low-reactivity sugars selected for controlled browning in prolonged processes.
Interactive Effects of Amino Acid and Sugar Combinations
The combinatorial effect of specific amino acid-sugar pairs generates distinctive flavor profiles and browning kinetics that cannot be predicted from individual component properties alone [75] [73]. Systematic investigation of these binary combinations provides insights into reaction specificity and enables targeted flavor development.
Optimization of Reactant Ratios
Experimental studies on Maillard reaction mixtures with varying concentration ratios reveal that 1:1 molar ratios typically generate optimal browning intensity [75]. For example, research on glucosamine-cyclohexylamine and glucosamine-benzylamine systems demonstrated maximum absorbance at 425 nm (indicating melanoidin formation) at equimolar concentrations when reacted at 120°C and pH 9.0 [75]. Significant deviation from this optimal ratio, such as 1:5 or 1:10 proportions, resulted in substantially reduced browning development [75].
Similar optimization patterns emerge in sugar-amino acid model systems. Glucose-lysine models exhibit peak browning at near-equimolar ratios, though the specific optimum varies with reaction conditions including temperature, pH, and water activity [75] [73]. This stoichiometric relationship reflects the mechanistic requirement for balanced nucleophile-electrophile pairing in the initial condensation step and subsequent rearrangement reactions.
Synergistic Flavor Development Pathways
The combination of specific amino acids with particular sugars generates signature flavor compounds through defined chemical pathways:
- Xylose-Lysine Systems: Produce roasted, meaty aromas through predominant formation of pyrazines and Strecker aldehydes [73].
- Ribose-Cysteine Systems: Generate characteristic meat-like flavors through sulfur-containing heterocycles (thiazoles, thiophenes) and furanthiols [2] [73].
- Fructose-Glycine Systems: Develop caramel-like, sweet aromatics through hydroxymethylfurfural (HMF) and maltol pathways [76] [77].
- Glucose-Tryptophan Systems: Yield floral, honey-like notes through indole and pyrazine derivatives [73].
These synergistic interactions demonstrate how precursor chemistry directs reaction trajectory toward specific sensory outcomes, providing a biochemical foundation for flavor prediction and design in food and pharmaceutical applications.
Experimental Approaches and Kinetic Analysis
Methodological standardization is essential for meaningful comparison of precursor reactivity across experimental systems. Well-established protocols enable quantitative assessment of how amino acid side chains and sugar types influence Maillard reaction progression and endpoint characteristics.
Standardized Experimental Protocol for Precursor Reactivity Assessment
Materials Preparation:
- Prepare 0.1M aqueous solutions of target reducing sugars (glucose, fructose, ribose, xylose) and amino acids (lysine, glycine, cysteine, histidine) in distilled water [75].
- Adjust initial pH to 9.0 using 0.1M NaOH or 0.1M HCl, verified with calibrated pH meter [75].
- Combine reactant solutions according to experimental design (typically varying concentration ratios from 10:1 to 1:10) in covered test tubes to prevent evaporation [75].
Reaction Conditions:
- Heat reaction mixtures at constant temperature (100-120°C) in dry oven for predetermined durations (0.5-3 hours) [75].
- Conduct reactions in triplicate to ensure statistical reliability [75].
- Terminate reactions by rapid cooling in ice bath [75].
Analytical Measurements:
- Measure browning intensity by absorbance at 420-425 nm using spectrophotometer, with appropriate dilutions to maintain optical density <1.0 [75].
- Quantify specific intermediates (HMF, furfural) via High-Performance Liquid Chromatography (HPLC) with UV detection [76] [77].
- Analyze volatile compounds by Solid-Phase Microextraction Gas Chromatography Mass Spectrometry (SPME-GC-MS) [77].
- Perform statistical analysis using two-way ANOVA with Fisher LSD test (significance level p<0.05) [75].
Kinetic Modeling of Precursor-Influenced Reactions
Kinetic modeling provides a powerful approach for quantifying precursor effects on Maillard reaction rates. Based on the general rate law, the disappearance of a reactant in a closed system follows:
[ -\frac{d[A]}{dt} = k[A]^n ]
where (k) represents the reaction rate constant and (n) the reaction order (typically (0 \leq n \leq 2)) [73]. The temperature dependence of reaction rates follows the Arrhenius equation:
[ k = k0 e^{-Ea/RT} ]
where (Ea) represents the activation energy, (R) the gas constant, and (T) absolute temperature [73]. Research demonstrates that both (Ea) and reaction order ((n)) vary significantly with precursor chemistry, explaining the divergent reactivity patterns observed with different amino acid-sugar combinations [73].
The following diagram illustrates the experimental workflow for systematic investigation of precursor effects in Maillard reactions:
The Scientist's Toolkit: Essential Research Reagents and Materials
Systematic investigation of precursor chemistry effects requires standardized research materials and analytical tools. The following table compiles essential reagents and their specific functions in Maillard reaction research.
Table 3: Essential Research Reagents for Investigating Precursor Chemistry in Maillard Reactions
| Reagent Category | Specific Examples | Research Function | Technical Considerations |
|---|---|---|---|
| Reducing Sugars | D-Glucose, D-Fructose, D-Ribose, D-Xylose, α-Lactose | Carbonyl source for reaction initiation; reactivity comparison | Purity >99%; prepare fresh solutions to avoid decomposition |
| Amino Acids | L-Lysine, L-Glycine, L-Cysteine, L-Histidine, L-Arginine | Nucleophilic amino group source; side chain functionality studies | Use free base forms; protect cysteine from oxidation |
| Amines | Cyclohexylamine, Benzylamine | Model nucleophiles for structure-reactivity studies | Handle in fume hood due to volatility and toxicity |
| Buffer Systems | Phosphate, Citrate, Carbonate buffers | pH control and optimization | Select buffers with minimal nucleophilic activity |
| Analytical Standards | 5-HMF, Furfural, Pyrazines, Nε-carboxymethyl-lysine (CML) | Quantification of specific reaction products | Purity >98%; prepare calibration curves for quantification |
| Chromatography Materials | C18 HPLC columns, SPME fibers, GC-MS columns | Separation and identification of reaction products | Method optimization required for different compound classes |
Implications for Food and Pharmaceutical Industries
Understanding precursor chemistry enables precise control of Maillard reaction outcomes in industrial applications. In food processing, targeted selection of amino acid-sugar combinations allows manufacturers to engineer specific flavor profiles, optimize color development, and mitigate formation of potentially harmful compounds [2] [74]. For instance, reducing free asparagine (the primary acrylamide precursor) or selecting low-reactivity sugar alternatives effectively limits acrylamide formation in baked goods [2] [74].
In pharmaceutical development, Maillard reaction control is crucial for preventing protein glycation in biotherapeutics and avoiding formation of advanced glycation end-products (AGEs) associated with diabetic complications and aging [48]. Strategic excipient selection, including amino acids that competitively inhibit protein glycation, represents a promising approach for stabilizing protein-based pharmaceuticals [48].
Recent research has also revealed beneficial applications of Maillard reaction products (MRPs), particularly their antibacterial properties against foodborne pathogens and even antibiotic-resistant strains [29]. Specific MRPs, including aminoreductones and glycated peptides, demonstrate broad-spectrum activity through mechanisms such as metal chelation, membrane disruption, and metabolic interference [29]. These findings suggest potential biomedical applications in medical device coatings and gut microbiota modulation [29].
The Maillard reaction represents a complex chemical network whose trajectory and outcomes are fundamentally dictated by precursor chemistry. The specific structural features of amino acid side chains—determining nucleophilicity, steric accessibility, and degradation pathway selection—interact with sugar redox potential and ring conformation to direct reaction course and product distribution. Quantitative understanding of these structure-reactivity relationships enables predictive control of Maillard chemistry across diverse applications, from food flavor optimization to therapeutic intervention against AGE-related pathologies. Future research should focus on elucidating molecular-level interactions between distinctive precursor combinations and developing kinetic models that incorporate the subtle steric and electronic effects governing reaction specificity.
This whitepaper synthesizes experimental evidence establishing a distinct hierarchy of reactivity among amino acids within the context of the Maillard reaction, a fundamental non-enzymatic browning process. The presented data, derived from model system studies, conclusively supports the order of Lysine > Cysteine > Isoleucine ≈ Glycine. This reactivity directly influences the chemodiversity of Maillard Reaction Products (MRPs) and the progression of browning, with significant implications for food chemistry, nutritional science, and the management of physiological glycation. The findings underscore the critical role of amino acid side-chain chemistry in directing reaction pathways and determining the composition of the resulting complex product mixture.
The Maillard reaction, a complex network of chemical interactions between carbonyl compounds (typically reducing sugars) and compounds bearing free amino groups (such as amino acids and proteins), is a cornerstone reaction in food science and physiology [1] [13]. Its products are responsible for the desired colors, flavors, and aromas in thermally processed foods, but are also implicated in the formation of potentially harmful substances and in vivo protein modifications linked to age-related diseases [6] [13]. A critical factor governing the trajectory and outcome of this reaction is the nature of the participating amino acid.
While all amino acids contain a primary amino group, their differing side chains, or R groups, confer vastly different chemical properties and reactivities. This review consolidates evidence from controlled model systems to demonstrate a clear reactivity hierarchy: Lysine > Cysteine > Isoleucine ≈ Glycine. This order is elucidated by monitoring the degradation of precursors, the formation of MRPs, and the development of browning, providing a predictive framework for understanding the Maillard reaction's course in more complex matrices.
Experimental Evidence for the Reactivity Hierarchy
The reactivity hierarchy is primarily established through studies employing equimolar amino acid-sugar model systems heated under controlled conditions. A pivotal study by Hellwig et al. utilized ribose and four different amino acids, monitoring the reaction over ten hours at 100°C using Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR-MS) to assign molecular formulae to the thousands of MRPs generated [6].
Table 1: Quantitative Summary of Maillard Reaction Product (MRP) Formation and Browning Intensity after 10 Hours in Ribose-Amino Acid Model Systems (100°C) [6]
| Amino Acid | Number of Distinct MRPs Detected | Relative Degree of Browning (294 nm) | Key Reactive Feature |
|---|---|---|---|
| Lysine | >700 | Highest | ε-Amino group on flexible side chain |
| Cysteine | 300-400 | Lowest | Nucleophilic thiol (-SH) group |
| Isoleucine | ~300 | Intermediate | Aliphatic, hydrophobic side chain |
| Glycine | ~300 | Intermediate | No side chain (hydrogen atom) |
The data in Table 1 reveals a direct correlation between the structural features of the amino acid and its reactivity. The order of total MRPs produced was Lysine > Cysteine > Isoleucine ≈ Glycine [6]. Interestingly, while cysteine produced a high number of MRPs, it resulted in the least amount of browning, highlighting how different amino acids can drive the Maillard reaction down distinct chemical pathways, some of which do not lead to colored melanoidins [6]. This dissociation between MRP chemodiversity and browning intensity is a key characteristic of cysteine's unique chemistry.
Further supporting evidence comes from a kinetic study investigating the reaction of glucose with essential amino acids across a range of pH values. This study reported that "Lysine was the most strongly destroyed amino acid," followed by threonine, further cementing lysine's position as the most reactive essential amino acid in non-enzymatic browning [78].
Detailed Methodologies for Key Experiments
The following section outlines the core experimental protocols used to generate the comparative data on amino acid reactivity.
FT-ICR-MS Analysis of MRP Chemodiversity
This protocol is adapted from the methodology used to generate the comprehensive data on MRP formation [6].
- Objective: To holistically identify and compare the number and type of non-volatile MRPs formed from different amino acids during the Maillard reaction.
- Reagents:
- 0.1 M D-Ribose solution
- 0.1 M solutions of L-Lysine, L-Cysteine, L-Isoleucine, and L-Glycine
- High-purity water and solvents (e.g., methanol) for MS analysis
- Procedure:
- Sample Preparation: Prepare equimolar (0.1 M) mixtures of ribose with each amino acid in separate vials. Adjust pH if studying its effect (unbuffered systems are used for fundamental reactivity comparisons).
- Thermal Treatment: Heat the sealed reaction vials at 100°C in a thermostated heating block or water bath. Remove aliquots at defined time intervals (e.g., 0, 2, 4, 6, 10 hours).
- Sample Quenching & Dilution: Immediately cool the aliquots on ice. Dilute samples appropriately with a solvent compatible with electrospray ionization (e.g., methanol/water) to stop the reaction and achieve a suitable concentration for mass spectrometry.
- Data Acquisition: Analyze the samples using direct-infusion FT-ICR-MS, typically in negative electrospray ionization [ESI(−)] mode to detect polar, oxygen-rich MRPs. The ultra-high mass resolution (>400,000 at m/z 300) is critical for separating the thousands of ion signals.
- Data Processing: Assign molecular formulae to the detected m/z-values using high mass accuracy (error < ±0.2 ppm) and isotopic fine structure validation. Filter results to include only features present in all analytical replicates.
Kinetic Monitoring of Browning and Precursor Loss
This method complements the FT-ICR-MS data by providing a classical assessment of reaction progress [78].
- Objective: To quantify the loss of reactants (sugar and amino acids) and the formation of colored products over time.
- Reagents:
- 0.1 M Glucose solution
- 0.1 M solutions of target amino acids
- Buffers for maintaining specific pH values (e.g., pH 4.0 to 12.0)
- Procedure:
- Reaction Setup: Prepare glucose-amino acid solutions in buffered media across a range of pH values.
- Heating: Heat the solutions at a constant temperature (e.g., 100°C).
- Sampling & Analysis:
- Browning Intensity: Measure absorbance of cooled aliquots at 294 nm (for intermediate MRPs) and 420 nm (for advanced, colored melanoidins) using a UV-Vis spectrophotometer.
- Precursor Loss: Quantify the remaining concentrations of glucose (e.g., via HPLC with refractive index detection) and amino acids (e.g., via HPLC with fluorescence detection after pre-column derivatization with OPA or similar) throughout the reaction.
Chemical Basis for the Reactivity Hierarchy
The observed reactivity is a direct consequence of the chemical properties of the amino acid side chains.
Lysine: The Most Reactive Amino Acid
Lysine's exceptional reactivity is attributed to the presence of a second, primary amino group on its side chain. The ε-amino group is more nucleophilic and less sterically hindered than the α-amino group, providing an additional and highly reactive site for the initial condensation with reducing sugars [6] [79]. This effectively doubles the potential for initiating the Maillard reaction compared to amino acids with only one amino group.
Cysteine: High Reactivity with Divergent Pathways
Cysteine possesses a highly nucleophilic thiol (-SH) group [80]. This group readily participates in the Maillard reaction, leading to the formation of a vast array of unique sulfur-containing MRPs, which accounts for the high number of detected compounds [6]. However, instead of proceeding predominantly through the classic browning pathways, cysteine often forms stable heterocyclic compounds (like thiazolidines) or traps reactive dicarbonyl intermediates as thioacetals, thereby suppressing the formation of brown melanoidins [6]. This explains the dichotomy of high MRP count but low browning.
Isoleucine and Glycine: Lower and Similar Reactivity
Isoleucine has a bulky, aliphatic, and hydrophobic side chain that offers no additional functional groups for reaction [79]. Its reactivity is driven solely by its α-amino group, which is sterically hindered by the adjacent side chain. Glycine, the simplest amino acid, has only a hydrogen atom as its side chain. While this means it has no steric hindrance, it also provides no additional functional groups to participate in or catalyze reactions [79]. Its reactivity is therefore limited to its α-amino group. The similar number of MRPs generated by isoleucine and glycine suggests that the steric hindrance of isoleucine and the lack of a functional side chain in glycine result in a comparable, and lower, overall reactivity in the Maillard reaction compared to lysine and cysteine [6].
Diagram 1: Amino acid side chains direct Maillard reaction pathways and outcomes. Lysine's ε-amino group drives diverse product formation and strong browning. Cysteine's thiol group leads to unique sulfur-containing products but suppresses browning. Isoleucine and glycine, with hindered or non-functional side chains, result in more limited product diversity.
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table details key reagents and instruments essential for conducting research on amino acid reactivity in the Maillard reaction, as featured in the cited studies.
Table 2: Key Research Reagents and Instrumentation for Maillard Reaction Studies [1] [6] [13]
| Item | Function / Rationale |
|---|---|
| Reducing Sugars (e.g., D-Ribose, D-Glucose) | Highly reactive carbonyl precursors. Ribose (a pentose) is often chosen for its high reactivity compared to hexoses. |
| Amino Acids (L-Lysine, L-Cysteine, L-Isoleucine, L-Glycine) | Amine precursors for model systems, allowing for the study of specific side-chain contributions. |
| pH Buffers | To control and investigate the profound effect of pH on Maillard reaction pathways and kinetics. |
| FT-ICR Mass Spectrometer | Provides ultra-high mass resolution and accuracy required for assigning molecular formulae to hundreds of MRPs in complex mixtures without prior separation. |
| HPLC Systems (with RID, UV/Vis, FLD) | For quantifying the loss of precursor sugars and amino acids over time during kinetic studies. |
| UV/Vis Spectrophotometer | Standard instrument for monitoring the development of browning by measuring absorbance at 294 nm and 420 nm. |
| Gas Chromatography-Mass Spectrometry (GC-MS) | Ideal for identifying and quantifying the volatile, often flavor-active MRPs (e.g., pyrazines, furans) generated during the reaction. |
The established reactivity hierarchy of Lysine > Cysteine > Isoleucine ≈ Glycine provides a fundamental chemical principle for predicting and controlling the Maillard reaction. This hierarchy has broad implications across multiple fields. In food science, it informs the selection of protein ingredients or additives to either enhance or suppress browning and flavor development in processed foods [1]. In nutritional science and medicine, the high reactivity of lysine explains why it is a major target for post-translational protein glycation in vivo, a process linked to diabetic complications and aging [81] [6]. Furthermore, understanding cysteine's ability to divert reaction pathways could inspire strategies to mitigate the formation of advanced glycation end-products (AGEs). Future research leveraging this hierarchy will be crucial for engineering healthier food profiles and developing interventions against glycation-related pathologies.
Non-enzymatic browning, particularly the Maillard reaction, is a complex network of chemical reactions that occurs between amino acids and reducing sugars during thermal processing of foods. This reaction is a double-edged sword: it is indispensable for developing desirable sensory properties such as flavor, aroma, and color in many cooked and processed foods, including bread, coffee, and chocolate [11] [2]. However, the same reaction pathway also leads to the formation of harmful compounds, notably acrylamide and Advanced Glycation End-products (AGEs), which pose significant health concerns [11] [82].
Acrylamide, a Group 2A probable human carcinogen, forms primarily in carbohydrate-rich foods processed at high temperatures (above 120°C) [83] [84]. Its formation is predominantly driven by a reaction between the amino acid asparagine and reducing sugars [83]. AGEs, such as N(ε)-carboxymethyllysine (CML) and pentosidine, are produced through analogous non-enzymatic reactions and can also be introduced exogenously through the diet [82]. These compounds are associated with oxidative stress, chronic inflammation, and the progression of various diseases, including diabetes, cardiovascular conditions, and neurodegeneration [82].
This whitepaper provides an in-depth technical guide for researchers and scientists, outlining the mechanisms of formation and evidence-based strategies to suppress acrylamide and undesirable AGEs. It is framed within a broader thesis on the chemistry of non-enzymatic browning, emphasizing practical, scalable interventions for the food industry and drug development sectors.
Mechanisms of Formation
The Maillard Reaction Pathway
The Maillard reaction is a three-stage process that initiates when a carbonyl group from a reducing sugar reacts with a nucleophilic amino group from an amino acid, peptide, or protein [11] [2].
- Stage 1 (Early Stage): The reaction begins with a condensation between the carbonyl group of a reducing sugar and an amino group to form an unstable Schiff base. This rapidly undergoes an Amadori rearrangement to form a more stable ketoamine compound [11] [2] [1].
- Stage 2 (Intermediate Stage): The Amadori products degrade into various reactive intermediates, including α-dicarbonyl compounds (e.g., glyoxal, methylglyoxal). A critical step at this stage is Strecker degradation, where α-dicarbonyls react with amino acids to produce Strecker aldehydes, which contribute to aroma [11] [1].
- Stage 3 (Advanced Stage): The final stage involves condensation, polymerization, and cyclization reactions of the intermediates, leading to the formation of high-molecular-weight, brown nitrogenous polymers called melanoidins, and other advanced products like AGEs [11] [1].
Acrylamide Formation
Acrylamide forms predominantly during the intermediate stage of the Maillard reaction. The primary pathway involves the amino acid asparagine reacting with a carbonyl source, typically a reducing sugar or an α-dicarbonyl compound [85] [84]. The reaction proceeds through a Schiff base intermediate, which decarboxylates to form an azomethine ylide. Acrylamide is then generated from this ylide via dehydration and deamination [85]. An alternative, less prominent route involves the formation of 3-aminopropionamide as an intermediate [85].
Figure 1: Primary Maillard pathway for acrylamide formation from asparagine.
Advanced Glycation End-products (AGEs) Formation
AGEs form endogenously in the body and exogenously in food through the Maillard reaction [82]. The initial steps mirror the early Maillard reaction, forming a Schiff base and then an Amadori product. Under oxidative stress, these early products can degrade into reactive α-dicarbonyl compounds (e.g., methylglyoxal, glyoxal). These highly reactive dicarbonyls then modify proteins, leading to the formation of irreversible, stable AGEs through processes of oxidation, dehydration, and cross-linking [82]. Common AGEs include CML, CEL, and pentosidine.
Figure 2: AGE formation pathway via protein glycation and oxidation.
Strategic Mitigation: Acrylamide
Formulation and Ingredient Strategies
Formulation adjustments target the primary precursors of acrylamide: free asparagine and reducing sugars.
Table 1: Formulation Strategies for Acrylamide Mitigation in Cereal-Based Products
| Strategy | Mechanism of Action | Application Example | Effect on Acrylamide |
|---|---|---|---|
| Use of Low-Asparagine Varieties | Reduces the primary nitrogen precursor for acrylamide formation. | Using wheat or rye varieties with low free asparagine content [83]. | Significant reduction potential; free asparagine concentration is the major determinant [83]. |
| Asparaginase Enzyme | Hydrolyzes free asparagine into aspartic acid, which is not an acrylamide precursor. | Application in dough for biscuits, crackers, and other cereal products [83]. | Can reduce acrylamide by >90% in model systems; effective in various food matrices [83]. |
| Addition of Certain Amino Acids | Competes with asparagine for reaction with carbonyl compounds. | Addition of L-cysteine or glycine to dough formulations [83] [85]. | Cysteine can reduce levels by >50%; effectiveness depends on concentration and food matrix [85]. |
| Reducing Reducing Sugards | Limits the carbonyl source required for the initial Maillard reaction. | Replacing fructose or invert sugar with sucrose in recipes [83]. | Can significantly reduce formation, as fructose is more reactive than glucose [83]. |
| Addition of Organic Acids | Lowers pH, protonating amino groups and reducing nucleophilicity. | Adding citric acid or lactic acid to dough [83]. | Reduction of ~30% or more; can impact leavening and flavor [83]. |
| Use of Alternative Leavening Agents | Avoids the use of ammonium bicarbonate, which enhances acrylamide formation. | Replacing ammonium bicarbonate with sodium bicarbonate in cookies and crisps [83]. | Can reduce acrylamide by >60%; may slightly alter product texture and color [83]. |
Source: Adapted from Formulation and Processing Strategies to Reduce Acrylamide... [83]
Processing and Operational Strategies
Optimizing processing conditions is critical for minimizing acrylamide without compromising food safety and quality.
- Temperature and Time Control: Lowering thermal processing temperatures and minimizing cooking time can dramatically reduce acrylamide. For instance, reducing biscuit baking temperature from 200°C to 180°C can decrease acrylamide by over 50% [83]. The principle of "thermal input control" is crucial—achieving the desired product color and moisture with the lowest possible time-temperature combination [83] [84].
- Fermentation Optimization: In yeast-fermented products like bread, extending fermentation time allows yeast to consume free asparagine and reducing sugars, thereby reducing precursors. Fermentation for 10-12 hours has been shown to effectively lower acrylamide levels in bread [83].
- Pre-Treatment of Raw Materials: Soaking or blanching potato slices in water before frying removes surface sugars, a key precursor. This simple step can significantly reduce acrylamide formation in French fries and potato chips [84]. Microwaving potatoes before frying has also been shown to reduce acrylamide by up to 40% [84].
Inhibition by Sulfur-Containing Compounds
Sulfur-containing compounds are highly effective inhibitors of acrylamide formation due to their strong nucleophilicity [85].
- Thiols (Cysteine, Glutathione): These compounds inhibit acrylamide through multiple mechanisms:
- Competitive Removal of Carbonyls: They compete with asparagine for reactive carbonyl and dicarbonyl intermediates [85].
- Nucleophilic Addition to Acrylamide: The thiolate anion (S-) reacts with already-formed acrylamide in a Michael-type addition, forming S-β-propionamide and effectively scavenging the toxicant [85]. The efficacy of cysteine is concentration-dependent; adding 1.5% cysteine to an asparagine/glucose model system resulted in non-detectable acrylamide levels [85].
- Other Sulfur Compounds: Sulfites (e.g., sodium bisulfite) and thiosulfinates (e.g., allicin from garlic) also act as effective inhibitors, though they may be less potent than thiols and can impart off-flavors [85].
Strategic Mitigation: Advanced Glycation End-products (AGEs)
Inhibiting Endogenous AGE Formation
The primary strategy for combating endogenous AGEs involves the use of compounds that trap reactive dicarbonyl intermediates or break existing AGE cross-links.
Table 2: Inhibitors of Advanced Glycation End-products (AGEs)
| Inhibitor Type | Example | Mechanism of Action | Research Findings / Experimental Use |
|---|---|---|---|
| Pharmaceutical Inhibitor | Aminoguanidine | Traps reactive α-dicarbonyl compounds (e.g., methylglyoxal, glyoxal) in the intermediate stage of glycation [86]. | First-known AGE inhibitor; associated with side effects (liver enzyme increase, dizziness) limiting clinical use [86]. |
| Natural Product Extract | Coptis chinensis (Huang Lian) | Exhibits dose-dependent inhibition of AGE formation; acts as a carbonyl trapper [86]. | In vitro studies show significant inhibition in NBT and Girard-T assays, comparable to positive controls [86]. |
| Natural Product Extract | Seriphium plumosum | Potent glycation inhibitor, likely through dicarbonyl trapping and antioxidant activity [86]. | Acetone crude extract of leaves showed 2.22% AGE formation vs. 7.4% for positive control (Arbutin) [86]. |
| Natural Product Extract | Bacopa monnieri | Inhibits AGE formation in a dose-dependent manner; mechanism may involve antioxidant and metal-chelating properties [86]. | Studied in vitro using the BSA-glucose model; effective across a range of concentrations [86]. |
| Synthetic Catalyst | Alagebrium (ALT-711) | Breaks pre-formed AGE-derived protein cross-links, restoring protein elasticity and function [82]. | Investigated in animal models and clinical trials for improving vascular stiffness in diabetic patients [82]. |
Source: Adapted from Natural Compounds as Inhibitors of Advanced Glycation... [86]
Reducing Dietary AGE Intake
Since a significant portion of the body's AGE burden comes from the diet, modifying food preparation methods is a key mitigation strategy [82].
- Cooking Method Selection: Cooking methods that use lower temperatures and higher moisture content (e.g., boiling, steaming, poaching) produce significantly fewer AGEs compared to high-temperature, dry-heat methods like grilling, frying, and roasting [82] [84].
- Marination with Acidic Solutions: Marinating foods in acidic ingredients (e.g., lemon juice, vinegar) before cooking can reduce AGE formation by creating a lower-pH environment, which slows the Maillard reaction [82].
- Process Optimization in Food Industry: The food industry can apply strategies similar to acrylamide mitigation, such as optimizing temperature-time profiles, using natural antioxidants, and selecting raw materials with lower precursor content to reduce AGE levels in processed foods [82].
Experimental Protocols for Inhibition Studies
In Vitro Protocol for Assessing Acrylamide Inhibition
This protocol is adapted from studies investigating the efficacy of sulfur-containing compounds like cysteine and glutathione [85].
Objective: To quantify the effectiveness of a potential inhibitor (e.g., L-Cysteine) in reducing acrylamide formation in a model system.
Materials:
- Reagents: L-Asparagine, D-Glucose, L-Cysteine, Phosphate Buffer (pH 7.0), Internal Standard for GC-MS/MS analysis.
- Equipment: Heating block or oil bath, Reactivials with Teflon-lined caps, GC-MS/MS system equipped with an ESI probe.
Procedure:
- Model System Preparation: Prepare a 10 mL aqueous solution in a reactivial containing 0.1 M L-Asparagine and 0.1 M D-Glucose in 0.1 M Phosphate Buffer (pH 7.0).
- Inhibitor Addition: Add the potential inhibitor (e.g., L-Cysteine) at the desired molar ratio relative to asparagine (e.g., 0.5:1, 1:1). Include a control vial with no inhibitor.
- Heat Treatment: Seal the vials tightly and heat in a heating block or oil bath at 160°C for 20 minutes.
- Rapid Cooling & Extraction: Immediately cool the vials in an ice-water bath to quench the reaction. Add a known amount of internal standard and extract acrylamide using a validated method (e.g., extraction with water, Carrez clarification, and solid-phase extraction cleanup).
- Analysis: Analyze the extracts using GC-MS/MS. Quantify acrylamide by comparing the peak area ratio of acrylamide to the internal standard against a calibration curve.
- Calculation: Calculate the percentage inhibition using the formula:
- Inhibition (%) = [1 - (Acrylamideinhibited / Acrylamidecontrol)] × 100
In Vitro Protocol for Assessing AGE Inhibition (BSA-Glucose Model)
This standard protocol is used to screen natural extracts and compounds for anti-glycation activity [86].
Objective: To evaluate the ability of a test compound (e.g., plant extract) to inhibit the formation of AGEs in a bovine serum albumin (BSA)-glucose model.
Materials:
- Reagents: Bovine Serum Albumin (BSA), D-Glucose, Sodium Azide (preservative), Phosphate Buffer Saline (PBS, pH 7.4), test compound/extract, Aminoguanidine (positive control).
- Equipment: Incubator (37°C), Fluorescence Spectrophotometer, Microplate reader.
Procedure:
- Incubation Mixture: Prepare the reaction mixture (1 mL total volume in PBS) containing 10 mg/mL BSA, 0.5 M D-Glucose, and 0.02% sodium azide.
- Test Compound Addition: Add the test compound/extract at various concentrations (e.g., 0.1, 0.5, 1.0 mg/mL). Include a negative control (BSA + Glucose only) and a positive control (BSA + Glucose + 1 mM Aminoguanidine).
- Incubation: Incubate all mixtures in the dark at 37°C for 1-3 weeks.
- Fluorescence Measurement: After incubation, measure the fluorescence of the formed AGEs using a fluorescence spectrophotometer (* λ_ex = 370 nm, λ_em = 440 nm*).
- Calculation: Calculate the percentage inhibition of AGE formation using the formula:
- Inhibition (%) = [1 - (Fluorescencesample / Fluorescencenegative_control)] × 100
- IC₅₀ Determination: Determine the concentration of the test compound that inhibits 50% of AGE formation (IC₅₀) by plotting inhibition % against log(concentration).
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagent Solutions for Studying Non-Enzymatic Browning Inhibition
| Research Reagent | Function in Experimental Protocols | Key Consideration |
|---|---|---|
| L-Asparagine | Primary precursor for acrylamide formation in model systems. | Purity is critical; use >99% purity for reproducible results in kinetic studies [85]. |
| D-Glucose / D-Fructose | Carbonyl source (reducing sugar) for Maillard reaction initiation. | Fructose is more reactive than glucose; choice impacts reaction rate and product profile [11] [85]. |
| L-Cysteine | Model thiol inhibitor for acrylamide studies; competes for carbonyls and traps acrylamide. | Can impart sulfurous odor at high concentrations; effectiveness is pH and temperature-dependent [85]. |
| Asparaginase Enzyme | Biological intervention to hydrolyze asparagine precursor in food matrices. | Activity and stability must be optimized for specific food pH and temperature conditions [83]. |
| Bovine Serum Albumin (BSA) | Model protein for in vitro glycation and AGE inhibition studies. | Fatty-acid-free BSA is preferred to avoid lipid oxidation interference [86]. |
| Aminoguanidine HCl | Positive control in AGE inhibition assays; a known dicarbonyl trapper. | Serves as a benchmark for comparing the efficacy of novel inhibitors [86]. |
| Methylglyoxal (MGO) / Glyoxal (GO) | Highly reactive α-dicarbonyl intermediates; used to study late-stage glycation and cross-linking. | Handling requires care due to high reactivity and toxicity; prepare fresh solutions [82]. |
The chemistry of the Maillard reaction presents a significant challenge: balancing the creation of desirable flavors with the suppression of harmful compounds like acrylamide and AGEs. A multifaceted, context-dependent approach is essential for effective mitigation. For acrylamide, this involves combining precursor reduction (e.g., asparaginase, selective breeding), process optimization (time-temperature control), and formulation interventions (amino acids, acids). For AGEs, strategies focus on inhibiting endogenous formation with synthetic or natural carbonyl traps and reducing dietary intake through modified cooking practices.
Future research should prioritize the development of highly effective, food-compatible inhibitors with minimal sensory impact. Exploring the synergistic effects of combined mitigation strategies and validating their efficacy in complex food matrices through advanced analytical techniques like LC-MS/MS and GC-MS/MS will be crucial. A deep, mechanistic understanding of non-enzymatic browning pathways remains the foundation for developing innovative solutions to enhance food safety and public health.
In the intricate chemistry of non-enzymatic browning and Maillard reaction research, pH stands as a fundamental regulatory parameter that exerts profound influence over reaction pathways and kinetics. The Maillard reaction, a complex network of chemical transformations between carbonyl compounds and amino groups, represents a critical area of study not only for food chemistry but also for pharmaceutical stability, where it can lead to drug degradation and formulation challenges. This technical guide examines the central role of pH as a master switch that controls nucleophile concentration and modulates reaction kinetics in these systems. Through its direct impact on the ionization states of reactive species, pH governs the availability of nucleophilic amines, affects the equilibrium between reducing sugar configurations, and ultimately determines the dominant reaction pathways and product distributions. Understanding these pH-mediated effects provides researchers with a powerful tool for predicting, controlling, and optimizing non-enzymatic browning processes across scientific and industrial applications.
Fundamental Mechanisms of pH Influence on Reaction Pathways
The Maillard reaction initiates when carbonyl groups of reducing sugars react with amino groups of amino acids, peptides, or proteins, culminating in the formation of brown pigments known as melanoidins [38] [87]. This complex reaction network proceeds through three primary stages: the initial formation of glycosylamines followed by Amadori rearrangement products; intermediate stages involving dehydration, fragmentation, and Strecker degradation; and final polymerization stages yielding heterogeneous brown polymers [38]. Throughout this cascade, pH serves as a master regulator that profoundly influences each stage through multiple mechanistic avenues.
The most direct influence of pH arises from its control over nucleophile concentration, specifically the proportion of amino groups in their reactive deprotonated state. At physiological and acidic pH values, amino groups exist primarily in their ammonium form (RNH₃⁺), which displays significantly reduced nucleophilicity compared to the free base form (RNH₂). The transition between these states occurs across the pH range according to the Henderson-Hasselbalch equation, with the inflection point centered at the pKa of the specific amino group involved. For typical amino acids and pharmaceutical amines, this pKa falls between 8 and 10, meaning that only at pH values exceeding these thresholds do significant concentrations of the reactive nucleophile become available [78]. This relationship establishes a fundamental kinetic barrier in mildly acidic and neutral environments, which can be overcome either by increasing pH or through alternative activation pathways that exhibit lower pH dependencies.
Beyond its effect on nucleophile concentration, pH dramatically influences the structural equilibrium of reducing sugars in solution. Reducing sugars exist in dynamic equilibrium between their cyclic hemiacetal forms and open-chain configurations, with the latter presenting the reactive carbonyl group necessary for initial Schiff base formation with amino groups. Under alkaline conditions, this equilibrium shifts toward the open-chain form through base-catalyzed ring opening, thereby increasing the concentration of the reactive carbonyl species and accelerating the initial condensation step [38]. Simultaneously, high pH conditions promote sugar fragmentation through caramelization pathways, even in the absence of amino compounds, generating highly reactive carbonyl intermediates that can subsequently participate in Maillard-type condensations [78]. This pH-dependent dual activation of both reaction partners establishes alkaline conditions as particularly conducive to rapid browning kinetics.
The degradation pathways of intermediate Maillard reaction products also exhibit significant pH dependence. The decomposition of Amadori rearrangement products proceeds through different mechanistic routes under acidic versus alkaline conditions, yielding distinct flavor-active and colored products. Under acidic conditions, 5-hydroxymethylfurfural (HMF) and furfural formation predominates, while alkaline conditions favor the formation of reductones and fission products that subsequently condense into nitrogen-containing heterocycles such as pyrazines and pyrroles [88] [38]. These pathway differentiations directly impact the sensory and functional properties of the resulting Maillard reaction product mixtures, with alkaline conditions generally producing more complex volatile profiles and intensified browning.
Table 1: pH-Dependent Reaction Pathways in Non-Enzymatic Browning
| pH Range | Dominant Reaction Pathways | Characteristic Products | Reaction Kinetics |
|---|---|---|---|
| Highly Acidic (pH < 4) | Ascorbic acid degradation, sugar dehydration | Furfural, HMF | Moderate browning, accelerated ascorbic acid degradation |
| Mildly Acidic (pH 4-6) | Schiff base formation, Amadori rearrangement | Early-stage intermediates | Slow browning, extended lag phase |
| Neutral (pH 6-8) | Multiple parallel pathways | Diverse intermediate products | Increased reaction rate, complex product mix |
| Alkaline (pH > 8) | Sugar fragmentation, Strecker degradation, condensation | Pyrazines, pyrroles, melanoidins | Rapid browning, minimal lag phase |
Quantitative Kinetic Data and pH Effects
The kinetic profile of non-enzymatic browning reactions demonstrates profound sensitivity to pH variations, with quantitative studies revealing dramatic accelerations under alkaline conditions. Research employing glucose-lysine model systems has demonstrated that reaction rates can increase by several orders of magnitude as pH transitions from acidic to alkaline ranges [89]. This acceleration follows a characteristic sigmoidal relationship when plotting reaction rate against pH, with the steepest increase occurring between pH 7 and 10, corresponding to the deprotonation of the ɛ-amino group of lysine (pKa ≈ 10.5) and the consequent increase in nucleophile concentration. Beyond this threshold, further pH elevation yields diminishing returns as the amino group approaches complete deprotonation, while simultaneously promoting competing caramelization pathways [78].
The degradation kinetics of individual Maillard precursors exhibits distinct pH dependencies that collectively determine the overall browning profile. In orange juice model systems stored at 42°C, reducing the pH from 3.8 to 1.5 significantly increased the degradation rate of ascorbic acid by up to 300% and dramatically enhanced the formation of furanic compounds including furfural and 5-hydroxymethylfurfural (HMF) [88]. These changes correlated directly with intensified browning, particularly during prolonged storage. Similarly, the presence of fructose as a reducing sugar enhanced HMF formation, while additional ascorbic acid accelerated overall browning kinetics across the pH spectrum, though the absolute rates remained strongly pH-dependent [88].
The temperature-pH interaction creates a complex kinetic landscape that determines practical reaction outcomes. Studies investigating the glucose-lysine system across pH values from 4.0 to 12.0 at 100°C demonstrated that lysine was the most susceptible essential amino acid to destruction under alkaline conditions, followed by threonine [78]. Notably, glucose degradation generally exceeded amino acid loss except under strongly alkaline conditions (pH > 9.0), where selective amino acid destruction became more pronounced. The kinetic data revealed that non-enzymatic browning generally followed pseudo-zero-order kinetics after an initial lag phase near neutrality, while reactant consumption displayed more complex patterns that deviated from simple first-order kinetics across the pH spectrum [78].
Table 2: Quantitative Kinetic Parameters as Functions of pH in Model Maillard Systems
| pH Value | Relative Browning Rate | Ascorbic Acid Degradation Rate (×10⁻³ day⁻¹) | HMF Formation Rate (×10⁻³ day⁻¹) | Lag Phase Duration (days) |
|---|---|---|---|---|
| 1.5 | 3.8 | 12.5 | 9.6 | 5 |
| 3.0 | 2.1 | 8.7 | 6.3 | 12 |
| 4.0 | 1.0 | 4.2 | 3.1 | 28 |
| 5.0 | 1.8 | 4.5 | 4.2 | 18 |
| 7.0 | 4.5 | 5.1 | 8.7 | 7 |
| 9.0 | 12.3 | 6.8 | 14.2 | 2 |
| 11.0 | 28.7 | 9.3 | 22.5 | <1 |
Experimental Protocols for Investigating pH Effects
Model System Preparation for pH-Dependent Maillard Reaction Kinetics
Establishing well-defined model systems represents a critical first step in quantifying pH effects on Maillard reaction kinetics. For liquid systems such as fruit juices or pharmaceutical solutions, researchers typically prepare model solutions containing precisely controlled concentrations of target reactants dissolved in buffered solutions. In orange juice studies, model systems incorporate ascorbic acid (0.5-1.0 mg/mL), fructose (20-50 mg/mL), and specific amino acids such as arginine (1-5 mg/mL) in buffered solutions ranging from pH 1.5 to 4.0 to simulate industrial storage conditions [88]. For broader pH profiling, phosphate buffers (pH 6.0-8.0) and carbonate buffers (pH 9.0-11.0) provide stable ionic environments, with all solutions adjusted to identical ionic strength to minimize secondary electrolyte effects. Following preparation, model solutions are transferred to sealed ampoules under nitrogen atmosphere to prevent oxidative side reactions, then subjected to controlled thermal acceleration typically at 40-45°C for shelf-life studies or 80-121°C for forced degradation protocols [88] [78].
Solid-state model systems employed in pharmaceutical and dry food applications utilize co-lyophilized or physically blended mixtures of reactive components with carefully controlled water activity (aw = 0.3-0.7). For drug-excipient compatibility studies, active pharmaceutical ingredients containing primary or secondary amines are blended with reducing sugars such as lactose or glucose in precise molar ratios (typically 1:1 to 1:3), with the mixture humidified to predetermined water activities using saturated salt solutions [87]. These solid systems are subsequently incubated at pharmaceutically relevant storage conditions (25°C/60% RH and 40°C/75% RH) with periodic sampling for analysis. The inclusion of appropriate controls, including non-reducing sugar analogs (e.g., mannitol instead of lactose) and amine-free systems, enables researchers to distinguish pH-specific effects from other degradation pathways.
Analytical Methodologies for Kinetic Parameter Determination
Comprehensive monitoring of Maillard reaction progression under varying pH conditions requires orthogonal analytical techniques targeting reactant consumption, intermediate accumulation, and brown pigment formation. High-performance liquid chromatography (HPLC) with UV/Vis and mass spectrometric detection provides quantitative data on the disappearance of parent amino acids and carbohydrates, while simultaneously tracking the formation of characteristic intermediates including furfurals, Amadori rearrangement products, and specific flavor compounds [88] [87]. For volatile aroma compounds characteristic of advanced Maillard reactions, gas chromatography-mass spectrometry (GC-MS) enables identification and quantification of pyrazines, furans, and sulfur-containing heterocycles across different pH conditions [38] [89].
Spectrophotometric methods offer complementary approaches for monitoring overall browning kinetics and specific indicator compounds. Ultraviolet-visible spectroscopy at 294 nm tracks the formation of intermediate compounds with conjugated double bonds, while measurement at 420 nm or 490 nm quantifies the development of brown melanoidin pigments [88] [78]. For pharmaceutical applications focused on early-stage Maillard reactions, LC-MS/MS methods targeting specific drug-sugar conjugates such as lactosylated drug products provide sensitive detection of initial reaction events before visible browning occurs [87]. Kinetic parameters derived from these analytical datasets, including rate constants, reaction orders, and activation energies, are subsequently modeled using appropriate mathematical approaches to extract pH-specific rate accelerations and pathway differentiations.
Research Reagent Solutions for pH-Controlled Maillard Studies
Table 3: Essential Research Reagents for pH-Controlled Maillard Reaction Studies
| Reagent Category | Specific Examples | Functional Role | pH Considerations |
|---|---|---|---|
| Buffer Systems | Phosphate (pH 6-8), Carbonate (pH 9-11), Citrate (pH 3-6) | Maintain constant pH environment | Ionic strength effects on reaction rates; buffer catalyzed reactions |
| Amino Compounds | L-Lysine, L-Arginine, Glycine, Pharmaceutical amines | Nucleophilic reaction partner | pKa determines reactive species concentration; side chain functionality influences pathway |
| Carbonyl Sources | D-Glucose, D-Fructose, L-Ascorbic acid, Lactose, Xylose | Electrophilic reaction partner | Ring-chain equilibrium pH dependence; fragmentation kinetics |
| Analytical Standards | 5-HMF, Furfural, Pyrazines, Amadori compounds | Quantification and method calibration | pH-dependent stability during analysis; extraction efficiency variations |
| Quenching Agents | Sodium borohydride, Methanol, Trichloroacetic acid | Arrest reactions at specific timepoints | Compatibility with analytical methods; pH adjustment requirements |
Pharmaceutical Implications and Formulation Strategies
The ramifications of pH-controlled Maillard chemistry extend significantly into pharmaceutical development, where drug-excipient incompatibilities present substantial stability challenges. Numerous active pharmaceutical ingredients containing primary or secondary amine functional groups undergo accelerated degradation when formulated with reducing sugars such as lactose, glucose, or maltose [87]. The protonation state of these amine groups, controlled by formulation pH, directly determines their nucleophilic capacity and consequently their reactivity toward carbonyl compounds. Case studies involving drugs such as pregabalin and fluoxetine demonstrate the formation of specific Maillard adducts, including Amadori rearrangement products, which accumulate under typical storage conditions and potentially compromise drug safety and efficacy [87].
Formulation strategies to mitigate undesired Maillard reactions leverage pH control as a primary intervention. For amine-containing drugs requiring solid dosage forms with enhanced stability, formulation scientists employ several protective approaches: (1) selection of non-reducing excipients such as cellulose derivatives, mannitol, or sucrose (non-reducing disaccharide) instead of lactose or glucose; (2) microenvironmental pH modification using acidic excipients to maintain the amine in its protonated, less nucleophilic state; (3) protective barrier technologies including film coating that physically separate reactive components; and (4) controlled moisture management through appropriate packaging, as water activity strongly influences Maillard reaction rates in solid formulations [87]. For liquid formulations, pH adjustment to the acidic range (typically pH 3-5) combined with refrigerated storage provides effective stabilization against Maillard degradation, though this must be balanced against potential acid-catalyzed hydrolysis pathways.
Accelerated stability studies for Maillard-prone formulations employ elevated temperature conditions (40°C/75% RH) to rapidly assess degradation trends, with careful attention to pH effects on predicted shelf-life. Kinetic modeling of pH-dependent degradation profiles enables rational formulation design by quantifying the trade-offs between Maillard reaction minimization and other stability considerations. For cases where pH adjustment proves insufficient, alternative strategies including antioxidant incorporation, oxygen-scavenging packaging, and lyophilization to reduce molecular mobility may provide complementary protection against drug-excipient interactions [87].
pH stands as a master regulatory parameter in non-enzymatic browning reactions, exerting multifaceted control over nucleophile availability, reaction pathway selection, and overall kinetic progression. Through its direct influence on the ionization state of amino groups and its indirect effects on sugar configuration equilibria, pH functions as a fundamental switch that directs the Maillard reaction toward distinct product profiles and rate accelerations. The quantitative relationships between pH and kinetic parameters, as characterized through carefully designed model systems and comprehensive analytical methodologies, provide researchers and formulators with predictive tools for controlling these complex chemical transformations. In pharmaceutical contexts, understanding and manipulating pH-dependent Maillard pathways becomes essential for ensuring drug stability and patient safety. Future research directions should focus on expanding quantitative kinetic databases across diverse chemical systems, developing advanced predictive models that incorporate pH effects, and designing novel intervention strategies that leverage pH control for optimal product outcomes across food, pharmaceutical, and biological applications.
Leveraging Water Activity and Reaction Time to Direct Pathways Towards Desired Endpoints
The Maillard reaction, a complex network of non-enzymatic browning reactions between carbonyl compounds and amino groups, profoundly influences the quality, safety, and nutritional profile of processed goods. For researchers and product developers, precise control over this reaction pathway is paramount. This technical guide elucidates the synergistic application of water activity (aₚ) and reaction time as critical levers to direct Maillard chemistry towards desired endpoints. Within product development, mastering these parameters enables the enhancement of sensory attributes while mitigating the formation of detrimental compounds such as acrylamide and advanced glycation end-products (AGEs) [11] [90].
The principle underpinning this control is that aₚ governs molecular mobility and reactant concentration, thereby influencing the reaction kinetics and the preferential formation of specific intermediates and end-products. Concurrently, the duration of thermal exposure determines the progression through the reaction's initial, intermediate, and final stages. This whitepaper provides a detailed examination of the underlying mechanisms, presents quantitative kinetic data, and offers validated experimental protocols for leveraging these parameters in research and industrial applications.
Fundamental Principles of Water Activity and Temporal Kinetics
The Role of Water Activity in Maillard Reaction Pathways
Water activity, defined as the ratio of the water vapor pressure of a substance to the vapor pressure of pure water at the same temperature, is a critical determinant of molecular mobility and reactivity. Its influence on the Maillard reaction is non-linear and optimal [11] [67]. At low aₚ levels (typically below 0.3), the reaction is severely limited as water, a product of the initial condensation stage, accumulates and shifts equilibrium backwards, inhibiting the reaction's initiation [67]. Furthermore, low aₚ often corresponds to a glassy state where high viscosity restricts the diffusion of reactants [91].
As aₚ increases, molecular mobility is enhanced through water's plasticizing effect. The reaction rate accelerates, reaching a maximum typically in the aₚ range of 0.65 to 0.75 [91] [92] [67]. Within this zone, the system provides sufficient molecular mobility for reactants to diffuse and interact, while the concentration of water is not yet high enough to significantly dilute the reactants or favor the reverse hydrolysis reactions. Beyond this maximum, further increases in aₚ lead to a dilution effect, where the concentration of amino groups and reducing sugars decreases, thereby slowing the reaction rate [11] [67].
Reaction Time and Progression through Maillard Stages
The Maillard reaction proceeds through distinct stages—initial, intermediate, and final—each characterized by the formation of specific compounds. The duration of thermal exposure, or reaction time, directly controls the progression through these stages [11] [93].
- Initial Stage: Involves the condensation of a reducing sugar with an amino group to form an N-substituted glycosylamine, which rearranges into a more stable Amadori compound. At this stage, there is no significant browning or fluorescence [11] [93].
- Intermediate Stage: The Amadori products degrade through various pathways, including dehydration, fragmentation, and Strecker degradation. This stage yields a multitude of volatile compounds (e.g., pyrazines, furans) that contribute to flavor and aroma, as well as colorless fluorescent compounds [11] [93].
- Final Stage: Characterized by condensation and polymerization reactions that produce high-molecular-weight, brown-colored melanoidins and advanced glycation end-products (AGEs) [11] [94] [93].
Prolonged reaction times drive the system towards the final stage, promoting the accumulation of brown pigments and high-molecular-weight polymers. Conversely, shorter times can preserve reactants or trap the system in the intermediate stage, favoring the formation of specific flavor compounds.
Quantitative Kinetics and Parameter Interplay
The interplay between water activity and reaction time is quantitatively described by reaction kinetics. The following table summarizes key kinetic parameters for Maillard reaction progression under varying aₚ conditions, derived from model and real food systems.
Table 1: Kinetic Parameters for Maillard Reaction under Different Water Activity Conditions
| System | Temperature (°C) | Optimal aₚ Range | Observed Reaction Kinetics | Key Findings |
|---|---|---|---|---|
| General Food Systems [67] | Varies | 0.65 - 0.75 | Pseudo-zero-order for browning | Maximum browning rate observed in this aₚ window. Rate decreases at higher aₚ due to reactant dilution. |
| Milk Powder Storage [91] | 20 - 50 | ~0.75 (for high aₚ impact) | Pseudo-first-order for lysine loss | Significant lysine loss (blockage) occurred primarily at high aₚ values, impacting nutritional quality. |
| Fish Powder Storage [67] | 20 - 50 | N.S. | Pseudo-zero-order for non-enzymatic browning | Browning rate was less affected by %RH (which controls aₚ) in the studied range (aₚ ~0.21-0.50) compared to oxidation. |
| Glucose-Lysine TTI Model [94] | 70 - 100 | N.A. (Liquid system) | Absorbance change over time | Activation energies (Eₐ) for browning of different TTI formulations ranged from 83.55 to 96.17 kJ mol⁻¹. |
Abbreviations: N.S. = Not Specified; N.A. = Not Applicable; TTI = Time-Temperature Indicator.
The temperature dependence of the reaction rate constant (k) is defined by the Arrhenius equation: k = k₀exp(-Eₐ/RT), where Eₐ is the activation energy, R is the universal gas constant, and T is the absolute temperature [94]. The Eₐ represents the sensitivity of the reaction to temperature changes. For instance, in Time-Temperature Indicators (TTIs) based on the Maillard reaction, Eₐ values can be tailored by selecting specific sugar-amino acid pairs, such as lysine and xylose, to match the activation energy of quality deterioration in target foods [94].
Table 2: Impact of Extended Reaction Time on Maillard-Derived Attributes in Food Systems
| System | Reaction Time | Impact on Maillard Reaction Endpoints |
|---|---|---|
| Coffee Roasting [92] | Shorter Maillard duration | Increased perceived sweetness and acidity; thinner body. |
| Coffee Roasting [92] | Longer Maillard duration | Muted acidity; increased buttery, milky, and mouth-coating viscosity. |
| Flour Products [90] | Excessive reaction time | Undesirable excessive browning, loss of essential amino acids (e.g., lysine), and formation of harmful compounds like acrylamide and 5-hydroxymethylfurfural (HMF). |
Experimental Protocols for Pathway Control
Protocol A: Determining the Optimal aₚ for Browning in a Model System
This protocol is designed to establish the relationship between water activity and the browning rate in a controlled model system, providing a foundation for product formulation.
- Objective: To quantify the rate of Maillard browning in a lysine-xylose model system across a range of water activities at a constant temperature.
- Materials:
- Amino Acid Solution: L-Lysine (0.25 M) in 0.2 M phosphate buffer (pH 7.0).
- Reducing Sugar Solution: D-Xylose (0.25 M) in the same buffer.
- Saturated Salt Solutions: Or humidity-controlled chambers for pre-equilibration (e.g., MgCl₂ for aₚ ~0.33, K₂CO₃ for ~0.43, Mg(NO₃)₂ for ~0.53, KI for ~0.69, KCl for ~0.84) [91] [94].
- Equipment: UV-Vis spectrophotometer, analytical balance, water bath, hermetic glass vials.
- Procedure:
- Prepare the reaction solution by mixing equal volumes of the lysine and xylose solutions.
- Dispense aliquots of the solution into multiple shallow glass vials.
- Pre-equilibrate the open vials in desiccators containing different saturated salt solutions at a constant temperature (e.g., 80°C) until constant weight is achieved, indicating aₚ equilibrium.
- Seal the vials and place them in an oven or water bath maintained at the target temperature (e.g., 80°C).
- At predetermined time intervals, remove triplicate vials from each aₚ condition.
- Dilute the samples appropriately with distilled water and measure the absorbance at 420 nm and/or fluorescence (e.g., excitation 347 nm, emission 415 nm) to monitor browning and fluorescent AGE formation [94] [93].
- Plot absorbance/fluorescence versus time for each aₚ to determine the reaction rate constant.
- Data Analysis: The aₚ condition yielding the highest rate of absorbance increase (slope) represents the optimal aₚ for browning under the given conditions. Fluorescence accumulation may follow different kinetics and should be analyzed separately [93].
Protocol B: Monitoring Lysine Blockage in a Proteinaceous Powder During Storage
This protocol is critical for evaluating the nutritional impact of the early Maillard reaction during the storage of low-moisture foods like protein powders.
- Objective: To determine the loss of bioavailable lysine due to lactosylation (or glycation with other sugars) in milk powder or a similar product during storage under different aₚ conditions.
- Materials:
- Skim milk powder or model protein powder.
- Saturated salt solutions for aₚ control (as in Protocol A).
- o-Phthaldialdehyde (OPA) reagent.
- Equipment: Spectrophotometer or fluorometer, desiccators, analytical balance.
- Procedure:
- Equilibrate powder samples to a range of target aₚ values using saturated salt solutions in desiccators at room temperature until equilibrium is reached [91].
- Seal the desiccators and store them at constant, mildly elevated temperatures (e.g., 20°C, 35°C, 50°C) to accelerate the reaction [91] [67].
- At regular time intervals, sample the powder and analyze for available lysine content using the OPA method [91]. The OPA reagent reacts with primary amines, and a decrease in absorbance/fluorescence compared to a control indicates lysine blockage.
- Data Analysis: Plot the natural logarithm of the percentage of remaining available lysine (ln(Lₜ/L₀)) versus time. A linear relationship indicates pseudo-first-order kinetics for lysine loss. The slope of the line is the rate constant (k) for each aₚ and temperature condition [91].
Visualization of Control Pathways and Workflows
The following diagram synthesizes the core concepts of this guide, illustrating how aₚ and time can be manipulated to direct the Maillard reaction towards specific sensory and compositional outcomes.
Diagram 1: Strategic control of Maillard reaction pathways through water activity and time. Low aₚ restricts progression, while medium aₚ enables full pathway development based on time. High aₚ dilutes reactants, slowing the overall process.
The experimental workflow for establishing these control parameters is outlined below.
Diagram 2: Generalized workflow for determining optimal aₚ and time parameters for a desired Maillard reaction outcome.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagent Solutions for Maillard Reaction Studies
| Reagent / Material | Function / Application | Example Use Case |
|---|---|---|
| L-Lysine & D-Xylose | Highly reactive model system for Maillard studies. | Forming a Maillard-based Time-Temperature Indicator (TTI) with tunable activation energy [94]. |
| Saturated Salt Solutions | Creating constant relative humidity environments for precise aₚ control during storage or equilibration. | Pre-equilibrating milk or fish powder samples to study the kinetics of lysine loss or browning during storage [91] [67]. |
| o-Phthaldialdehyde (OPA) Reagent | Spectrophotometric/Fluorometric quantification of primary amines, specifically available lysine. | Monitoring the loss of bioavailable lysine due to the early Maillard reaction in protein-rich systems [91]. |
| Phosphate Buffers (e.g., 0.2 M, pH 6.5-8.0) | Controlling pH, a key parameter that influences the concentration of nucleophilic amino groups. | Investigating the effect of pH on the initial condensation step of the Maillard reaction in liquid model systems [11] [94]. |
| Pronase E (Protease) | Enzymatic digestion of proteins to release protein-bound Maillard reaction products for analysis. | Detecting fluorescent AGEs in instant soy milk powder or other proteinaceous foods [94]. |
The deliberate manipulation of water activity and reaction time provides a powerful, fundamental methodology for steering the complex pathways of the Maillard reaction. By understanding the non-linear relationship between aₚ and reaction rate, and by quantitatively mapping the progression of chemical changes over time, scientists can predictably optimize processes to achieve specific quality attributes. Whether the goal is to maximize flavorful volatiles, minimize nutritional loss, control color development, or reduce harmful compounds, the strategic application of the principles and protocols outlined herein is essential. Future research will continue to refine these kinetic models, particularly for multi-component real-food systems, and explore the synergistic effects of other parameters like pH and reactant composition to enable even more precise control over this cornerstone of food chemistry.
Comparative Pathways and Clinical Validation: From Food Models to Disease Biomarkers
Non-enzymatic browning reactions, particularly the Maillard reaction, represent a network of complex chemical processes that significantly impact food quality, nutritional value, and product stability. These reactions occur between carbonyl groups of reducing sugars and nucleophilic groups of amino acids, peptides, or proteins, leading to the formation of numerous compounds that influence color, flavor, and aroma. Understanding the relative reactivity of different sugars is crucial for controlling these reactions in food processing and pharmaceutical development. This review provides a comparative analysis of the reactivity of fructose versus glucose in Maillard reaction pathways and examines the potential inhibitory role of sorbitol, a sugar alcohol commonly used as an alternative sweetener. The kinetic parameters and reaction pathways differ substantially between these sugars, leading to varied rates of browning and formation of advanced glycation end-products. Recent research has also revealed unexpected complexities in the behavior of sorbitol, traditionally considered non-reactive, when combined with amino acids under thermal processing conditions.
Fundamental Mechanisms of Non-Enzymatic Browning
The Maillard Reaction Pathway
The Maillard reaction is a complex network of reactions that can be divided into three primary stages: initial, intermediate, and final. The process begins with a nucleophilic addition reaction between a carbonyl group of a reducing sugar and a free amino group of an amino acid or protein, forming a Schiff base. This intermediate rapidly undergoes Amadori rearrangement to form more stable ketoamine compounds known as Amadori products [11] [2]. The specific pathway and rate of this initial stage are highly dependent on the sugar structure, with fructose and glucose demonstrating distinct rearrangement patterns and reaction kinetics.
In the intermediate stage, Amadori products undergo various degradation pathways influenced by pH conditions. Under acidic conditions (pH ≤ 7), these products primarily undergo 1,2-enolization, leading to the formation of furfurals such as hydroxymethylfurfural (HMF) from hexoses. Under alkaline conditions (pH > 7), 2,3-enolization is favored, producing reductones and fission products [1]. These reactive intermediates can then undergo Strecker degradation with amino acids, producing characteristic aroma compounds and α-dicarbonyl compounds, which are key precursors to browning.
The final stage involves condensation and polymerization reactions of various intermediates, leading to the formation of heterogeneous, high molecular weight, brown-colored nitrogenous polymers known as melanoidins [1]. These compounds are responsible for the characteristic brown color associated with the advanced Maillard reaction.
Caramelization and Ascorbic Acid Degradation
Beyond the Maillard reaction, non-enzymatic browning can also occur through caramelization and ascorbic acid degradation. Caramelization involves the direct thermal decomposition of sugars without the participation of amino compounds, while ascorbic acid can oxidize to dehydroascorbic acid and subsequently participate in browning reactions [95]. These pathways often operate concurrently with Maillard reactions in complex food systems, contributing to the overall browning phenomenon.
The following diagram illustrates the core mechanistic pathways of non-enzymatic browning, highlighting the key stages and compounds involved:
Figure 1: Fundamental Pathways of Non-Enzymatic Browning
Comparative Reactivity: Fructose vs. Glucose
Structural Basis for Differential Reactivity
The fundamental difference in reactivity between fructose and glucose stems from their structural characteristics. Glucose is an aldose sugar, featuring an aldehyde group, while fructose is a ketose sugar with a ketone group. This structural distinction significantly influences their reaction pathways and kinetics in Maillard reactions. The open-chain form of fructose is more prevalent in solution compared to glucose, increasing its availability for nucleophilic addition reactions [11]. Furthermore, the ketose structure of fructose facilitates different rearrangement patterns following the initial Schiff base formation, leading to distinct downstream reaction products.
Fructose typically demonstrates greater reactivity in Maillard reactions compared to glucose. This enhanced reactivity is attributed to several factors: the greater proportion of open-chain form in equilibrium, increased susceptibility to dehydration reactions, and the formation of more reactive intermediates during the reaction pathway [96]. These characteristics make fructose more prone to participate in both early and advanced stages of the Maillard reaction, resulting in accelerated browning and formation of advanced glycation end-products.
Experimental Evidence from Model Systems
Recent research utilizing model systems and food matrices has provided quantitative evidence for the differential reactivity of fructose and glucose. A comprehensive study investigating non-enzymatic browning in guava fruit leather demonstrated significant differences between sugar types across multiple parameters [95]. The following table summarizes key experimental findings comparing fructose and glucose reactivity:
Table 1: Comparative Analysis of Fructose vs. Glucose Reactivity in Guava Fruit Leather Model System
| Parameter | Fructose | Glucose | Analytical Method |
|---|---|---|---|
| Browning Index | Higher | Lower | Spectrophotometric measurement at 440 nm |
| Non-Enzymatic Browning | 0.42 ± 0.02 | 0.31 ± 0.01 | OD at 440 nm after ethanol extraction |
| HMF Content (ng/g) | 32.32 ± 1.5 | 18.45 ± 0.9 | HPLC with UV detection at 280 nm |
| Furfural Content (ng/g) | 0.95 ± 0.05 | 0.62 ± 0.03 | HPLC with UV detection at 280 nm |
| Antioxidant Activity Retention | Lower | Moderate | FRAP assay (mg AAE/100g) |
| Ascorbic Acid Degradation | Higher | Moderate | 2,6-dichlorophenol indophenol method |
| Total Free Amino Acids Consumption | Higher | Moderate | Ninhydrin method (mg leucine/100g) |
The experimental data clearly indicates that fructose generates significantly higher levels of advanced Maillard reaction products, particularly hydroxymethylfurfural (HMF), which serves as a key indicator of sugar degradation and browning progression. The concentration of HMF in fructose-containing systems (32.32 ng/g) was approximately 75% higher than in glucose-containing systems (18.45 ng/g) [95]. This substantial difference in HMF formation directly correlates with the observed browning intensity and confirms the enhanced reactivity of fructose in non-enzymatic browning pathways.
Kinetic and Mechanistic Considerations
The reaction kinetics of fructose and glucose follow distinct patterns due to their different degradation pathways. Fructose predominantly forms Heyns products upon rearrangement, while glucose forms Amadori products [1]. These different rearrangement products subsequently degrade via distinct pathways, leading to varied distributions of α-dicarbonyl compounds and other reactive intermediates. Fructose-derived Heyns products tend to degrade more rapidly, generating higher concentrations of reactive dicarbonyl intermediates such as 3-deoxyglucosone, which accelerate the formation of brown pigments [96].
The pH of the reaction medium significantly influences the degradation pathways of both sugars. Under acidic conditions, fructose more readily undergoes dehydration to form HMF, while glucose requires higher activation energy for this conversion [11]. This difference contributes to the observed enhanced browning potential of fructose in intermediate-moisture food systems and pharmaceutical formulations where acidic conditions may prevail.
Sorbitol: From Putative Inhibitor to Unexpected Reactant
Traditional Understanding of Sorbitol in Browning Reactions
Sorbitol, a sugar alcohol derived from the hydrogenation of glucose, has traditionally been considered non-reactive in Maillard reactions due to its lack of a carbonyl group. Without this essential functional group, sorbitol cannot form the initial Schiff base with amino groups, thus theoretically preventing initiation of the classic Maillard reaction pathway [97]. This structural characteristic has led to the widespread use of sorbitol as a browning inhibitor in sugar-free food products and pharmaceutical formulations where color stability is paramount.
Experimental evidence from model systems appeared to support this inhibitory role. Research on guava fruit leather demonstrated that sorbitol-added samples exhibited significantly lower browning parameters compared to reducing sugar-containing systems [95]. Specifically, sorbitol-containing samples showed a browning index of only 0.15 ± 0.01, compared to 0.42 ± 0.02 for fructose and 0.31 ± 0.01 for glucose. Similarly, HMF formation in sorbitol systems (12.8 ng/g) was less than half that observed in glucose systems and approximately 40% of that in fructose systems [95].
Unexpected Browning in Sorbitol-Amino Acid Systems
Contrary to traditional understanding, recent investigations have revealed that sorbitol can participate in browning reactions when heated with amino acids. This surprising phenomenon challenges conventional wisdom regarding the chemical stability of sugar alcohols in food and pharmaceutical systems [97] [98]. A study examining the browning reaction in a sorbitol/glycine model system demonstrated significant browning development upon thermal processing, with absorbance at 420 nm (browning index) increasing up to 7.42-fold in the presence of glycine compared to sorbitol-alone systems [97].
The mechanism underlying this unexpected browning involves the glycine-promoted conversion of sorbitol to reducing sugars, primarily glucose, which subsequently participate in classical Maillard reaction pathways [97]. Quantitative analysis revealed that glucose concentration in glycine-containing systems was 63.2 times higher than in systems without glycine after heating. This conversion occurs through a series of dehydration and oxidation reactions, leading to the formation of reactive intermediates that initiate browning pathways.
Mechanistic Pathways for Sorbitol Conversion
The transformation of sorbitol to reactive carbonyl compounds occurs through specific degradation pathways promoted by the presence of amino acids. The primary mechanism involves the dehydration of sorbitol to form anhydrosugar alcohols, particularly 1,4-anhydrosorbitol (1,4-AHSO), 1,5-anhydrosorbitol (1,5-AHSO), and isosorbide (ISO) [98]. These dehydration products subsequently undergo oxidation or further rearrangement to form reducing sugars such as glucose and fructose, which then participate in standard Maillard reaction pathways with available amino acids.
The following diagram illustrates the complex transformation pathway of sorbitol when heated with amino acids:
Figure 2: Sorbitol Transformation and Browning Pathway with Amino Acids
Research has demonstrated that the presence of glycine significantly promotes sorbitol degradation, with higher glycine dosages accelerating the formation of dehydration products. The promotion effect is most pronounced for 1,5-AHSO, whose concentration increases dramatically with increasing glycine dosage [98]. This catalytic effect of amino acids on sorbitol degradation represents a previously overlooked pathway for browning in sugar-free products containing both sorbitol and amino acids.
Experimental Methodologies for Investigating Sugar Reactivity
Model System Design and Preparation
The investigation of sugar reactivity in non-enzymatic browning typically employs model systems that simplify complex food matrices while maintaining chemical relevance. For comparative studies of fructose and glucose reactivity, a common approach involves preparing solutions of each sugar at equivalent molar concentrations (typically 0.1-1.0 M) in appropriate buffers (e.g., phosphate buffer, pH 5.0-8.0) with added amino acids (commonly glycine, lysine, or a protein such as casein) [95] [97]. These model systems allow for controlled investigation of specific reaction pathways without interference from other food components.
For sorbitol reactivity studies, model systems typically involve preparing aqueous solutions of sorbitol (1-2 M) with added glycine or other amino acids at various molar ratios [97] [98]. The solutions are then subjected to controlled heating at temperatures relevant to food processing (60-120°C) for specified time periods. To establish comparative controls, parallel systems containing sorbitol alone, amino acids alone, and traditional reducing sugars with amino acids are typically prepared and processed simultaneously.
Analytical Techniques for Monitoring Browning
Multiple analytical techniques are employed to monitor the progression of non-enzymatic browning and quantify reaction products:
Browning Intensity: Measured by spectrophotometric analysis of absorbance at 420 nm for brown pigments and 294 nm for intermediate compounds [97]. Samples are typically diluted appropriately and measured against solvent blanks.
HMF and Furfural Quantification: Using reverse-phase HPLC with UV detection at 280-285 nm [95]. Separation is commonly achieved using C18 columns with mobile phases consisting of water-acetonitrile or water-methanol mixtures, often with added modifiers such as tetrahydrofuran.
α-Dicarbonyl Compounds Analysis: Quantified using GC-MS or HPLC following derivatization with o-phenylenediamine (OPD) [97] [98]. This approach allows for sensitive detection of key intermediates such as glyoxal, methylglyoxal, and 3-deoxyglucosone.
Sugar and Sorbitol Analysis: Monitoring substrate depletion and product formation using HPLC with refractive index detection or GC-MS after appropriate derivatization [97].
Color Measurements: Using colorimetry in the CIE Lab* color space to quantify visual color changes, with calculation of browning indices based on specific formulae incorporating these coordinates [95].
Key Research Reagents and Materials
Table 2: Essential Research Reagents for Investigating Sugar Reactivity in Non-Enzymatic Browning
| Reagent/Material | Function/Application | Technical Specifications |
|---|---|---|
| D-Fructose | Reducing sugar substrate for Maillard reaction studies | High purity (>99%), analytical standard grade |
| D-Glucose | Reducing sugar for comparative reactivity studies | Anhydrous, high purity (>99.5%) |
| Sorbitol | Sugar alcohol for inhibition/transformation studies | Pharmaceutical grade, purity ≥99.8% |
| Glycine | Model amino acid for Maillard reaction studies | Analytical grade, purity ≥99% |
| o-Phenylenediamine (OPD) | Derivatization agent for α-dicarbonyl compounds | HPLC grade, suitable for derivatization |
| HPLC Standards | Quantification of reaction products | Certified reference materials (HMF, furfural, etc.) |
| Potassium Phosphate Buffer | pH control in model systems | Analytical grade, various pH values (5.0-8.0) |
| Citric Acid | Acidulant for pH adjustment in model systems | Food grade, anhydrous |
| Methanol/Acetonitrile | HPLC mobile phase components | HPLC grade, low UV absorbance |
Implications for Food and Pharmaceutical Applications
The differential reactivity of fructose and glucose has significant implications for product formulation in both food and pharmaceutical industries. Formulators must consider the browning potential of sugar selections, particularly for products requiring extended shelf-life or thermal processing. The enhanced reactivity of fructose may be desirable in certain applications where rapid color and flavor development are sought (e.g., baking, caramel production), but problematic in others where color stability is paramount (e.g., clear beverages, light-colored sauces, pharmaceutical syrups).
The unexpected browning behavior of sorbitol in the presence of amino acids presents both challenges and opportunities for product development. For sugar-free products where color stability is essential, reformulation strategies may be necessary to separate sorbitol from protein components or incorporate competitive inhibitors that block the catalytic effect of amino acids on sorbitol degradation [98]. Alternatively, understanding these reaction pathways enables better prediction of shelf-life and color development in products containing sorbitol-amino acid combinations.
In pharmaceutical development, particularly for liquid formulations and protein-based therapeutics, the insights from sugar reactivity studies inform excipient selection and storage condition optimization. The potential for Maillard reaction-induced protein modification must be carefully considered when formulating with reducing sugars or polyols like sorbitol, especially for products requiring long-term stability [39].
This comparative analysis demonstrates the complex reactivity patterns of fructose, glucose, and sorbitol in non-enzymatic browning pathways. Fructose exhibits significantly greater reactivity compared to glucose, leading to accelerated browning and higher yields of advanced reaction products such as HMF. Meanwhile, sorbitol, traditionally considered a non-reactive browning inhibitor, demonstrates unexpected transformation pathways when heated with amino acids, leading to browning through conversion to reducing sugars. These findings have profound implications for product formulation, processing optimization, and shelf-life prediction in food and pharmaceutical systems. Future research should focus on elucidating the precise catalytic mechanisms through which amino acids promote sorbitol degradation and developing targeted strategies to control these reactions in complex product matrices.
Within non-enzymatic browning and Maillard reaction research, a central challenge is connecting observable macroscopic changes with precise molecular-level transformations. Traditional spectrophotometric indices, specifically absorbance at 294 nm (A~294~) and 420 nm (A~420~), have long served as empirical indicators for monitoring the formation of intermediate and advanced-stage Maillard reaction products (MRPs), respectively [11]. While these indices are invaluable for tracking reaction kinetics, they lack the resolution to identify specific chemical entities. The advent of ultra-high resolution mass spectrometry, particularly Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR-MS), provides the capability to resolve the complex molecular composition of MRPs [99]. This technical guide details methodologies for the systematic correlation of FT-ICR-MS data with traditional browning indices, thereby validating model systems and creating predictive frameworks for advanced Maillard reaction research.
Theoretical Foundation: Bridging Macroscopic and Molecular Data
The Significance of Traditional Browning Indices
The Maillard reaction is a complex network of chemical processes that can be broadly divided into stages. Spectrophotometric measurements at specific wavelengths serve as proxies for tracking progress through these stages [11].
- Absorbance at 294 nm (A~294~): This index primarily measures the formation of colorless intermediate compounds, including furfurals and other carbonyl-containing products that absorb in the UV spectrum. These compounds are characteristic of the early and intermediate stages of the Maillard reaction.
- Absorbance at 420 nm (A~420~): This index measures the development of brown pigments known as melanoidins. These high-molecular-weight, nitrogen-containing polymers are the hallmark of the advanced, final stages of the Maillard reaction [11].
The Analytical Power of FT-ICR-MS
FT-ICR-MS offers unparalleled mass accuracy and resolution, allowing for the assignment of unique molecular formulas (e.g., C~c~H~h~N~n~O~o~) to thousands of individual compounds within a complex MRP mixture without prior chromatography [99]. The key to correlation lies in interpreting these molecular formulas in terms of their chemical characteristics and reaction pathways.
Research has successfully linked optical properties to molecular composition using rank correlation to connect parameters like A~294~ and A~420~ to the elemental formulas derived from FT-ICR-MS [99]. This approach allows scientists to determine, for instance, that compounds removed during a specific process (like coagulation) which absorb at higher wavelengths, significantly correlate with formulas that are more oxidized and have lower H/C ratios [99].
Experimental Protocols
Generating and Sampling Maillard Reaction Model Systems
- Model System Design: Prepare model systems relevant to your research (e.g., glucose/glycine for simple systems; glucose/lysine or fructose/casein for more complex ones). Dissolve reactants (typically 0.1-1.0 M) in a suitable buffer (e.g., 0.1 M phosphate buffer, pH 7.4).
- Controlled Heating: Heat the reaction solutions in sealed vials or reactors at a constant temperature (e.g., 80°C to 140°C) for a defined time series (e.g., 0, 30, 60, 120 minutes).
- Sampling and Quenching: At each time point, immediately withdraw aliquots and rapidly cool them in an ice bath to quench the reaction.
Measuring Traditional Browning Indices
- Sample Preparation: Dilute reaction aliquots appropriately with distilled water to ensure absorbance readings fall within the linear range of the spectrophotometer (typically A < 1.5).
- Spectrophotometric Measurement:
- Using a UV-Vis spectrophotometer, measure the absorbance of the diluted sample at 294 nm (A~294~) and 420 nm (A~420~) against a blank of distilled water or buffer [13].
- Use a 1 cm pathlength quartz cuvette.
- Record measurements in triplicate and calculate the mean and standard deviation.
FT-ICR-MS Sample Preparation and Analysis
- Sample Clean-up and Dilution: To prevent instrument contamination and ionization suppression, desalt and purify samples using solid-phase extraction (e.g., C18 cartridges) or dialysis. Dilute the processed samples to a consistent dissolved organic carbon concentration with a high-performance liquid chromatography (HPLC)-grade solvent, typically 50:50 methanol:water [99].
- Data Acquisition: Analyze samples using an FT-ICR-MS system equipped with an electrospray ionization (ESI) source, operating in either positive or negative ion mode.
- Critical MS Settings:
- Mass Range: 100 - 1000 Da
- Resolution: > 400,000 at m/z 400
- Number of Scans: 64-128 accumulations to improve signal-to-noise ratio.
- Critical MS Settings:
- Data Processing: Use specialized software (e.g., Bruker DataAnalysis, Composer) to calibrate mass spectra internally and assign molecular formulas (C~c~H~h~N~n~O~o~S~s~) to each observed peak. Apply conservative constraints (e.g., element ranges: C 0-50, H 0-100, O 0-30, N 0-5) and accept only formulas with an assignment error of < 0.5 ppm.
Data Integration and Correlation Methodology
The core of the validation process involves statistically linking the molecular data from FT-ICR-MS to the kinetic data from the browning indices.
Molecular Characterization from Assigned Formulas
From the assigned molecular formulas, calculate these key molecular descriptors for each time point:
- Hydrogen to Carbon Ratio (H/C): An indicator of aromaticity and unsaturation. Lower H/C ratios (< 1.0) are associated with more condensed, aromatic structures typical of melanoidins [99].
- Oxygen to Carbon Ratio (O/C): An indicator of oxidation state. Higher O/C ratios are often linked to more polar, carboxylic acid-rich intermediates.
- Average Carbon Oxidation State (OS~C~): A composite index calculated as OS~C~ ≈ 2 × (O/C) - (H/C). This helps distinguish between reduced (low OS~C~) and oxidized (high OS~C~) material [99].
- Double Bond Equivalent (DBE): A measure of the total number of rings and double bonds in a molecule.
Statistical Correlation Analysis
- Data Matrix Construction: Create a data matrix where rows represent individual molecular formulas detected across all time points, and columns include their molecular descriptors (H/C, O/C, OS~C~, DBE) and their relative abundances (or presence/absence) at each time point.
- Correlation with Indices: Perform a non-parametric rank correlation analysis (e.g., Spearman's Rho) between the relative abundance of each molecular formula and the measured values of A~294~ and A~420~ at corresponding time points [99].
- Interpretation: Molecular formulas showing a strong positive correlation (e.g., Spearman's ρ > 0.8, p < 0.01) with A~420~ are likely constituents of the brown melanoidin pigments. Those correlating with A~294~ are likely intermediate compounds, such as furans or carbonyl-containing species.
The following workflow diagram illustrates the complete experimental and analytical pipeline from model system preparation to final correlation and validation.
Key Research Reagent Solutions and Materials
Table 1: Essential research reagents and materials for correlating FT-ICR-MS data with browning indices.
| Reagent / Material | Function / Rationale |
|---|---|
| Reducing Sugars (e.g., D-Glucose, D-Fructose, Lactose) | Carbonyl group donors; the type of sugar influences reaction rate and product profile [11]. |
| Amino Acids/Proteins (e.g., Glycine, Lysine, β-Lactoglobulin) | Amino group donors; lysine residues are primary targets, influencing both flavor and nutritional value [11] [39]. |
| Buffer Salts (e.g., Potassium/Sodium Phosphate) | Maintains precise pH control, a critical parameter as it influences the concentration of nucleophilic amino groups [11]. |
| Solid-Phase Extraction (SPE) Cartridges (e.g., C18, HLB) | Desalting and purification of MRPs prior to FT-ICR-MS analysis to prevent ionization suppression and instrument contamination [99]. |
| HPLC-Grade Solvents (Methanol, Water) | Dilution medium for FT-ICR-MS analysis; high purity is essential to avoid background contamination [99]. |
Data Interpretation and Application
The correlation analysis allows for a molecular-level interpretation of the traditional browning indices, as summarized in the table below.
Table 2: Interpretation of molecular descriptors and their correlation with traditional browning indices.
| Molecular Descriptor | Correlation with A~294~ (Intermediates) | Correlation with A~420~ (Melanoidins) | Chemical Implication |
|---|---|---|---|
| H/C Ratio | Moderate negative correlation | Strong negative correlation [99] | Lower H/C indicates higher unsaturation/aromaticity in melanoidins. |
| O/C Ratio | Variable / Weak positive | Strong positive correlation [99] | Higher O/C indicates extensive oxidation in colored end-products. |
| OS~C~ (Oxidation State) | Moderate positive correlation | Strong positive correlation [99] | Confirms that melanoidin formation is linked to carbon oxidation. |
| N-containing Formulas | Low to moderate prevalence | High prevalence and abundance [99] | Confirms incorporation of nitrogen from amino acids into polymers. |
This validated, correlated model system provides a powerful tool for:
- Predictive Modeling: Forecasting browning kinetics based on initial reactant composition.
- Process Optimization: In food and pharmaceutical industries, guiding processes to maximize desirable flavors and colors while minimizing harmful compounds like acrylamide [11].
- Health Research: Identifying specific Maillard reaction products in vivo that may be linked to diabetes and age-related diseases [39].
The Maillard reaction, a form of non-enzymatic browning, represents a fundamental chemical process characterized by reactions between reducing sugars and amino groups. While the core chemical mechanisms are conserved, the environment in which these reactions occur—whether in thermally processed foods or living biological systems—dictates vastly different pathways, kinetics, and health consequences. In food systems, the Maillard reaction is harnessed to develop desirable sensory attributes (flavors, aromas, colors) but can also generate detrimental compounds such as acrylamide and advanced glycation end-products (AGEs). In biological systems, the analogous reaction occurs endogenously, leading to the formation of AGEs which are implicated in the pathophysiology of diabetes, cardiovascular disease, and other age-related chronic conditions. This whitepaper provides an in-depth technical comparison of the Maillard reaction across these two domains, detailing shared chemical mechanisms, contrasting environmental conditions, analytical methodologies for monitoring reaction progression, and the divergent implications for human health and disease. The objective is to furnish researchers and drug development professionals with a consolidated framework for understanding this critical biochemical process and its multifaceted impact.
The Maillard reaction, first described by Louis Camille Maillard in 1912, is a pervasive form of non-enzymatic browning [30] [10]. It is defined by a complex network of chemical interactions between carbonyl groups (primarily from reducing sugars) and nucleophilic amino groups (from amino acids, peptides, or proteins) [2]. This reaction is singular in its significance, spanning the domains of food science and medicine. As noted by chemistry Nobel Prize winner Jean-Marie Lehn, "The Maillard is, by far, the most widely practiced chemical reaction in the world" [10].
The fundamental chemistry initiating the reaction sequence is conserved: a carbonyl group reacts with a trivalent nitrogen atom [100]. However, the environment in which this reaction occurs—characterized by parameters such as temperature, pH, water activity, and time—diverges dramatically between a sizzling steak and a living cell. In food systems, the reaction is often driven by high-temperature processing and is integral to developing the characteristic flavors and colors of baked goods, roasted coffee, and grilled meat [30] [10]. In biological systems, the same fundamental reaction proceeds slowly at physiological temperatures, leading to the gradual accumulation of advanced glycation end-products (AGEs), which are associated with the aging process and the pathogenesis of several chronic diseases [30] [1] [2]. This whitepaper dissects the similarities and contrasts between these two worlds, providing a technical guide for professionals navigating the implications of this critical reaction pathway.
Core Chemical Mechanism: A Shared Foundation
The Maillard reaction mechanism proceeds through three canonical stages, consistent across both food and biological contexts, though the specific reactants and intermediates can vary [30] [1] [2]. The following diagram illustrates this shared chemical pathway.
Stage 1: Initial Condensation and Rearrangement
The reaction initiates with a nucleophilic addition between the carbonyl group of a reducing sugar (e.g., glucose, fructose) and a free amino group (e.g., from lysine) to form a reversible Schiff base [1] [2]. This intermediate rapidly undergoes an Amadori rearrangement (for aldose sugars) or a Heyns rearrangement (for ketose sugars) to form more stable Amadori or Heyns rearrangement products, respectively (e.g., lactulosyllysine in milk) [30] [1]. This stage is reversible and does not produce color.
Stage 2: Intermediate Diversification and Fragmentation
The Amadori/Heyns products undergo diverse pathways, including dehydration, fragmentation, and retro-aldol reactions, leading to the formation of highly reactive dicarbonyl compounds (e.g., deoxyosones, methylglyoxal) [1] [6]. These dicarbonyls are pivotal intermediates. They can subsequently participate in Strecker degradation with amino acids, producing Strecker aldehydes and aminoketones, which are key precursors for aroma compounds [1] [2]. The specific pathway is heavily influenced by pH.
Stage 3: Polymerization and Brown Pigment Formation
The final stage involves the condensation and polymerization of the various intermediates from the second stage (e.g., furfurals, reductones, dicarbonyls) [30]. This leads to the formation of heterogeneous, high-molecular-weight, brown-colored nitrogenous polymers known as melanoidins in foods and as advanced glycation end-products (AGEs) in biological systems [30] [1]. While the core structure of these polymers is poorly characterized, they represent the terminal, irreversible products of the reaction cascade.
Contrasting Environments and Parameters
Despite the shared chemical foundation, the environmental conditions in food versus biological systems dictate profoundly different reaction kinetics, pathways, and outcomes. The following table summarizes the key contrasting parameters.
Table 1: Environmental and Parametric Contrasts Between Food and Biological Systems
| Parameter | Food Systems | Biological Systems |
|---|---|---|
| Primary Driver | Thermal processing (e.g., baking, frying, roasting) [10] | Endogenous metabolic activity; hyperglycemia [30] |
| Typical Temperature | High (e.g., 140–165°C / 280–330°F) [10] | Low (37°C / 98.6°F) [30] |
| Reaction Timescale | Short (minutes to hours) [30] | Very long (years to decades) [30] |
| pH Environment | Can vary widely (acidic to alkaline); often manipulated to control browning [10] [2] | Narrowly regulated near neutral (pH ~7.4); pathological shifts can occur locally |
| Water Activity (aw) | Variable; low-moisture foods favor advanced stages [30] [2] | High and constant (aw ≈ 1.0) |
| Primary Reactants | Free amino acids, peptides, proteins, various reducing sugars [6] | Long-lived proteins (e.g., collagen, crystallins), nucleic acids, membrane lipids; intracellular sugars (e.g., glucose, fructose-6-phosphate) |
| Key Modulators | Metal ions (can catalyze), antioxidants (can inhibit) [2] | Enzymatic defense systems (e.g., glyoxalase), antioxidant molecules |
Food System Environment
In food processing, the Maillard reaction is primarily extrinsic and engineerable. High temperatures are applied to achieve desired sensory properties rapidly [10]. Parameters like pH and water activity are actively controlled; for instance, an alkaline environment (e.g., lye on pretzels) deprotonates amino groups, increasing their nucleophilicity and accelerating browning [10]. The choice of cooking method (e.g., grilling vs. boiling) significantly impacts the profile of Maillard Reaction Products (MRPs) formed, particularly for compounds like heterocyclic amines (HCAs) in meat [30].
Biological System Environment
In contrast, the reaction in vivo is an intrinsic, slow biochemical process often referred to as "glycation" [30] [1]. It occurs continuously at physiological temperature and pH, driven by the concentration of reducing sugars and the half-life of the target biomolecule. Hyperglycemia, as seen in diabetes, is a major accelerant of this process [30]. Biological systems have evolved complex defense mechanisms, including enzymatic systems like the glyoxalase pathway that detoxify reactive dicarbonyl intermediates and receptor-mediated clearance of AGEs. The failure of these systems with age or disease leads to pathological accumulation.
Analytical Methodologies for Reaction Monitoring
A suite of sophisticated analytical techniques is employed to monitor the Maillard reaction's progression and products, with method selection depending on the specific stage or analyte of interest. The following workflow outlines a multi-technique approach for comprehensive analysis.
Detailed Experimental Protocols
4.1.1 Protocol: Quantification of Early-Stage MRPs (Furosine) by HPLC
- Principle: Furosine is an acid hydrolysis product of the Amadori compound lactulosyllysine and is a marker for early Maillard reaction in dairy products and pasta [30] [13].
- Procedure:
- Hydrolysis: Weigh 100 mg of sample (e.g., milk powder, pasta) into a hydrolysis tube. Add 5 mL of 7.6 N hydrochloric acid. Seal tube under vacuum and hydrolyze at 110°C for 24 hours.
- Sample Clean-up: Cool the hydrolysate and filter. Dilute the filtrate with ultrapure water. Pass an aliquot through a solid-phase extraction (SPE) C18 cartridge. Elute furosine with a water/methanol mixture.
- HPLC Analysis: Inject the eluent into an HPLC system equipped with a C18 reverse-phase column and a UV or DAD detector (wavelength: 280 nm). Use an isocratic or gradient mobile phase of water with low concentrations of an ion-pairing agent (e.g., heptafluorobutyric acid) and acetonitrile.
- Quantification: Identify furosine by retention time matching with an authentic standard. Construct a calibration curve with known concentrations of the standard for quantification [30].
4.1.2 Protocol: Non-targeted Analysis of MRPs using FT-ICR-MS
- Principle: Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR-MS) provides ultra-high mass resolution and accuracy, enabling the assignment of molecular formulae to thousands of compounds in complex MRP mixtures without prior separation [6].
- Procedure:
- Model System Preparation: React equimolar (e.g., 0.1 M) solutions of a reducing sugar (e.g., ribose) and an amino acid (e.g., glycine, lysine) in a buffer or water. Heat the mixture at a controlled temperature (e.g., 100°C) for varying durations (e.g., 2, 4, 6, 10 hours) [6].
- Sample Dilution: After reaction, cool the samples and dilute appropriately with a suitable solvent (e.g., methanol/water 1:1 v/v) for mass spectrometry.
- Direct Infusion MS: Introduce the diluted sample directly into the FT-ICR-MS instrument via a syringe pump or flow injection, using electrospray ionization (ESI) in positive or negative mode.
- Data Processing: Acquire mass spectra with high resolution (>400,000 at m/z 300). Use software to perform internal calibration, peak picking, and molecular formula assignment (considering C, H, O, N, S elements). Utilize van Krevelen diagrams and compositional networks to visualize chemical space and propose reaction pathways based on elemental composition changes (e.g., dehydration, decarboxylation) [6].
The Scientist's Toolkit: Key Research Reagent Solutions
Table 2: Essential Reagents and Materials for Maillard Reaction Research
| Reagent/Material | Function and Application in Research |
|---|---|
| Reducing Sugars (D-Glucose, D-Ribose, D-Fructose, Lactose) | Primary carbonyl donors in model systems. Ribose is highly reactive, useful for accelerated studies; Lactose is key for dairy product research [6]. |
| Amino Acids (L-Lysine, L-Glycine, L-Cysteine, L-Arginine) | Primary amino group donors. Lysine is highly reactive due to its ε-amino group; Cysteine leads to distinct sulfur-containing flavors and can suppress browning [6] [2]. |
| Buffer Solutions (e.g., Phosphate, Carbonate) | To control and maintain pH, a critical parameter influencing reaction pathways (e.g., 1,2- vs. 2,3-enolization) [1]. |
| Analytical Standards (HMF, Furosine, CML, Acrylamide) | Certified reference materials for targeted quantification using HPLC-MS or GC-MS, essential for method validation and accurate reporting [30] [1] [13]. |
| Solid-Phase Extraction (SPE) Cartridges (C18, Ion-Exchange) | For sample clean-up and pre-concentration of analytes from complex food or biological matrices (e.g., urine, tissue hydrolysates) prior to analysis [13]. |
| Deuterated Solvents (D₂O, CD₃OD) | For NMR-based structural analysis of MRPs and reaction pathways [13]. |
| Enzymes (Asparaginase) | Investigational reagent for mitigating harmful MRPs; asparaginase reduces acrylamide formation by converting the precursor asparagine to aspartic acid [10]. |
Consequences and Health Implications
The outcomes of the Maillard reaction are dichotomous, presenting both beneficial and detrimental effects, with the balance heavily dependent on the system and extent of reaction.
Consequences in Food Systems
- Desirable Effects:
- Sensory Attributes: Generation of characteristic aromas (from pyrazines, furans, thiophenes), tastes (umami, savory), and colors (brown melanoidins) in foods like bread, coffee, chocolate, and roasted meat [10] [100] [2].
- Antioxidant Activity: Some MRPs, particularly melanoidins, exhibit antioxidant properties, which can improve food stability and potentially confer health benefits [30] [2].
- Detrimental Effects:
- Nutritional Loss: The reaction can make essential amino acids, especially lysine, biologically unavailable, reducing protein quality [30] [101].
- Toxicant Formation:
- Acrylamide: Forms from asparagine and reducing sugars at high temperatures (>120°C) and is a classified probable human carcinogen [10] [1].
- Heterocyclic Amines (HCAs): Generated from creatine/creatinine, amino acids, and sugars in well-done muscle meats; associated with increased cancer risk [30].
- Advanced Glycation End-products (AGEs): Dietary AGEs, such as Nε-(carboxymethyl)lysine (CML), are consumed through processed foods and are implicated in promoting oxidative stress and inflammation when absorbed [30] [1].
Consequences in Biological Systems
The consequences in vivo are predominantly pathological, stemming from the accumulation of AGEs.
- Protein Dysfunction: AGEs can cross-link long-lived structural proteins like collagen and elastin, leading to reduced elasticity in blood vessels, skin, and tendons [30] [1].
- Cellular Signaling Disruption: AGEs exert their pathological effects largely by binding to the Receptor for AGE (RAGE). This binding activates several detrimental signaling pathways, as illustrated below.
- Disease Associations: The AGE-RAGE axis is a well-established contributor to the complications of diabetes (e.g., nephropathy, retinopathy), cardiovascular disease (atherosclerosis), neurodegenerative disorders (Alzheimer's disease), and the general aging process [30] [1].
The following table provides a consolidated summary of these contrasting consequences.
Table 3: Contrasting Consequences of the Maillard Reaction in Food vs. Biological Systems
| Aspect | Food Systems | Biological Systems |
|---|---|---|
| Primary Benefits | Sensory Quality: Flavor, aroma, color development [10] [100].Functionality: Antioxidant activity of some melanoidins [30] [2]. | (Primarily none, though endogenous glycation is an unavoidable consequence of metabolism) |
| Primary Detriments | Nutritional: Loss of protein bioavailability (e.g., lysine) [30].Safety: Formation of process contaminants (acrylamide, HCAs) [30] [10]. | Pathological: Protein cross-linking and dysfunction [1].Cellular: Oxidative stress, chronic inflammation via RAGE [30].Disease: Diabetes complications, cardiovascular disease, aging [30] [1]. |
| Key Example Compounds | Desirable: Melanoidins (color), 2-acetyl-1-pyrroline (aroma) [10].Undesirable: Acrylamide, CML, Heterocyclic Amines [30] [2]. | Pathological AGEs: Nε-(carboxymethyl)lysine (CML), Pentosidine, Methylglyoxal-derived hydroimidazolone (MG-H1) [30] [1]. |
The Maillard reaction stands as a paradigm of how a fundamental chemical process can manifest with profoundly different implications across disparate environments. The shared core mechanism—the reaction between carbonyls and amines—belies the critical importance of context. In food systems, it is a rapid, high-energy process managed for sensory and preservation outcomes, albeit with risks from thermal toxicants. In biological systems, it is a slow, insidious process contributing to the molecular basis of aging and chronic disease.
Future research efforts must continue to bridge these two worlds. In food science, the development of novel processing technologies and interventions (e.g., precision heating, enzymatic pre-treatments like asparaginase, natural inhibitors) to minimize harmful MRPs while preserving desirable qualities is a key priority [10] [13]. In medicine and pharmacology, the focus remains on elucidating the precise structures of pathogenic AGEs, understanding the full spectrum of RAGE signaling, and developing therapeutic agents that can inhibit AGE formation, break existing cross-links, or block RAGE activation [1]. The continued application of advanced analytical techniques like FT-ICR-MS and high-resolution LC-MS will be crucial for mapping the complex "Maillard reactome" in both contexts, leading to more targeted and effective strategies for managing its consequences in human health and disease.
Cysteine presents a compelling paradox in Maillard reaction (MR) research: it demonstrates high reactivity yielding a diverse array of Maillard reaction products (MRPs) while simultaneously suppressing the characteristic browning development. This whitepaper synthesizes current scientific understanding to resolve this paradox by elucidating the unique chemical pathways initiated by cysteine's sulfhydryl group. Through detailed analysis of reaction mechanisms, quantitative product profiling, and experimental methodologies, we establish how cysteine redirects traditional Maillard pathways toward sulfur-containing flavor compounds and colorless adducts at the expense of melanoidin formation. The insights gained from cysteine's behavior provide a framework for manipulating MR pathways in food science and pharmaceutical development to achieve targeted product profiles while mitigating undesirable browning or toxicant formation.
The Maillard reaction constitutes a complex network of chemical interactions between amino compounds and carbonyl compounds, significantly impacting food quality, flavor chemistry, and physiological processes. Within this reaction framework, cysteine stands as a notable exception to general amino acid behavior. While typically classified among the more reactive amino acids in MR systems, cysteine consistently produces significantly less browning compared to other amino acids like lysine under identical conditions [6]. This creates an apparent contradiction: high chemical reactivity coupled with low visual browning.
Research utilizing Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) has revealed that cysteine-ribose model systems generate 300-400 distinct MRPs after 10 hours at 100°C, a number significantly exceeding those produced by glycine or isoleucine and approaching the diversity observed with lysine [6]. Despite this molecular diversity, spectrophotometric measurement at 420 nm (indicative of advanced-stage browning) shows cysteine producing minimal browning compared to other amino acids [6]. This paper systematically deconstructs this paradox by examining the unique chemical mechanisms privileged by cysteine's sulfhydryl group and their implications for controlling non-enzymatic browning in industrial applications.
Quantitative Evidence: Mapping the Paradox
Empirical data from model systems clearly demonstrates the divergent behavior of cysteine in Maillard reaction contexts. The following tables summarize key quantitative comparisons that define the cysteine paradox.
Table 1: Comparative MRP Formation and Browning Intensity in Amino Acid-Ribose Model Systems (10 hours at 100°C) [6]
| Amino Acid | Number of Distinct MRPs | Browning Intensity (A420 nm) | Reactivity Order |
|---|---|---|---|
| Lysine | >700 | High | 1 (Highest) |
| Cysteine | 300-400 | Very Low | 2 |
| Isoleucine | 300-400 | Medium-High | 3 |
| Glycine | 300-400 | Medium | 3 |
Table 2: Key Flavor Compounds Generated in Cysteine-Xylose-Glycine Model System [102]
| Compound Class | Specific Compounds Identified | Potential Aroma Attributes |
|---|---|---|
| Sulfur-containing | 2-Furfurylthiol, 3-Mercapto-2-pentanone, 2-Methyl-3-furanthiol, 2-Acetylthiazole | Meaty, roasted, sulfurous |
| Thiophenes | 2-Methylthiophene, 3-(Methylthio)thiophene | Meaty, roasted |
| Pyrazines | Pyrazine, 2,6-Dimethylpyrazine, Ethylpyrazine | Nutty, roasted |
| Furanones | Dihydro-2-methyl-3(2H)-furanone | Caramel-like |
Mechanistic Insights: Sulfhydryl-Directed Pathway Divergence
The resolution to the cysteine paradox lies in the unique nucleophilicity and redox activity of its sulfhydryl group, which redirects reaction fluxes toward alternative pathways. The following diagram illustrates the key branching points in cysteine-involved Maillard reactions.
Competitive Thiazolidine Formation
In the initial Maillard stage, cysteine preferentially forms 2-threityl-thiazolidine-4-carboxylic acid (TTCA) through cyclization between its sulfhydryl group and the carbonyl group of reducing sugars [102]. This stable, colorless cyclic compound represents a competitive pathway that depletes the precursor pool available for browning pigment formation. TTCA formation is reversible and acid-catalyzable, slowly releasing intermediates that proceed to form sulfur-containing flavor compounds while continuing to bypass strong browning pathways [102].
Dicarbonyl Trapping and Redirected Flux
During the intermediate Maillard stage, highly reactive α-dicarbonyl intermediates (e.g., glyoxal, methylglyoxal) are generated, which normally polymerize to form melanoidins. Cysteine effectively traps these dicarbonyl compounds through several mechanisms:
- Thioacetal Formation: The sulfhydryl group reacts with carbonyl groups to form colorless thioacetals, preventing their polymerization into pigments [33].
- HMF Sequestration: 5-Hydroxymethylfurfural (HMF), a key intermediate in browning pathways, reacts with cysteine to form 1-dicysteinethioacetal-5-hydroxymethylfurfural (DCH), a colorless adduct [33]. This reaction competitively inhibits HMF's participation in melanoidin formation.
- Strecker Degradation Modification: Cysteine participates in modified Strecker degradation pathways that generate sulfur-containing flavor compounds like thiophenes, thiazoles, and furanthiols instead of brown nitrogenous polymers [103].
These trapping mechanisms explain both the high MRP diversity (through numerous diversion pathways) and the suppressed browning (through prevention of pigment polymerization).
Experimental Protocols: Methodological Framework
FT-ICR-MS Analysis of MRP Diversity
Objective: To comprehensively characterize the molecular diversity of MRPs generated in cysteine-containing model systems [6].
Protocol:
- Model System Preparation: Prepare equimolar (0.1 M) solutions of L-cysteine and ribose in phosphate buffer (pH 7.4). Use individual component solutions (cysteine alone, ribose alone) as controls.
- Thermal Treatment: Heat solutions at 100°C for 2, 4, 6, and 10 hours in sealed ampules. Quench reactions in ice water.
- Sample Preparation: Dilute samples 1:100 (v/v) in methanol:water (1:1) containing 0.1% formic acid. Centrifuge at 14,000 × g for 10 minutes before analysis.
- FT-ICR-MS Analysis:
- Instrument: Fourier Transform Ion Cyclotron Resonance Mass Spectrometer
- Ionization: Negative electrospray ionization (ESI-) mode
- Mass Range: m/z 100-600
- Resolving Power: 400,000 at m/z 300
- Mass Accuracy: <0.2 ppm
- Data Processing:
- Use compositional network-based formula annotation
- Apply isotopic fine structure validation
- Generate van Krevelen diagrams for visualization
- Classify compounds as MRPs, sugar degradation products, or amino acid degradation products
HMF-Cysteine Adduct Characterization
Objective: To identify and characterize the reaction product between HMF and cysteine that suppresses browning [33].
Protocol:
- Adduct Synthesis: React HMF (5 mM) with L-cysteine (10 mM) in phosphate buffer (75 mM, pH 7.4) at 80°C for 2 hours.
- HPLC-DAD Analysis:
- Column: Agilent Eclipse XBD-C18 (4.6 × 250 mm, 5 μm)
- Mobile Phase: A) 30 mM ammonium formate buffer (pH 2.44); B) Acetonitrile
- Gradient: 5-30% B over 20 minutes
- Detection: 235 nm, 254 nm, and 284 nm
- Column Temperature: 35°C
- LC-MS/MS Identification:
- Use electrospray ionization in positive mode
- Monitor for m/z 385.1 [M+H]+ corresponding to DCH
- Perform MS/MS fragmentation for structural confirmation
- NMR Validation:
- Purify compound using preparative HPLC
- Analyze using 1H NMR and 13C NMR in D2O
- Confirm thioacetal structure through characteristic proton signals
Kinetic Analysis of Browning Inhibition
Objective: To quantitatively measure cysteine's inhibition of Maillard browning and relate it to reaction conditions [104].
Protocol:
- Model System: Prepare fructose-glutamic acid system (0.2 M each) in phosphate buffer (75 mM, pH 7.4) with varying cysteine concentrations (0-20 mM).
- Thermal Processing: Heat at 80°C for 9 hours in water bath with periodic sampling.
- Browning Measurement:
- Monitor absorbance at 420 nm (final stage browning) and 294 nm (intermediate MRPs)
- Use microplate reader for high-throughput analysis
- HMF Quantification:
- Employ HPLC with diode array detection
- Use external HMF standard curve (0.015625-2.0 mg/mL)
- Monitor at 284 nm for maximum HMF sensitivity
- Data Analysis:
- Calculate browning inhibition percentage relative to control
- Establish correlation between cysteine concentration and HMF reduction
- Determine kinetic parameters for browning suppression
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for Cysteine-Maillard Reaction Research
| Reagent/Chemical | Function in Research | Specific Application Notes |
|---|---|---|
| L-Cysteine | Primary amino acid precursor | Use >98% purity; store at -4°C under nitrogen when possible |
| D-Ribose | Highly reactive pentose sugar | Enables rapid MRP formation at moderate temperatures |
| D-Xylose | Pentose for meat flavor studies | Ideal for sulfur-containing flavor compound generation |
| 5-Hydroxymethylfurfural (HMF) | Key intermediate for trapping studies | Standard for quantifying browning pathway activity |
| Phosphate Buffer (pH 7.4) | Physiological pH model system | 75 mM concentration optimal for browning studies |
| Deuterated DMSO | NMR solvent for structural elucidation | Essential for characterizing novel MRP adducts |
| Methanol with 0.1% Formic Acid | ESI-MS sample preparation | Enhances ionization efficiency for polar MRPs |
| Kojic Acid | Reference tyrosinase inhibitor | Comparative agent for enzymatic browning studies |
Research Implications and Applications
Flavor Modulation and Food Safety
The unique pathway diversion caused by cysteine enables targeted generation of desirable meaty and roasted flavor compounds while suppressing browning and potentially harmful advanced glycation end-products (AGEs). Studies demonstrate that cysteine effectively inhibits formation of heterocyclic aromatic amines like PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine), a potent carcinogen, by competitively reacting with precursor phenylacetaldehyde [103]. This presents strategic opportunities for developing cleaner label flavor systems and safer thermally processed foods.
Pharmaceutical and Therapeutic Applications
Understanding cysteine's Maillard modulation mechanisms provides insights for controlling protein glycation in biological systems. The trapping of reactive dicarbonyl species mirrors physiological defense mechanisms against AGE accumulation, suggesting potential therapeutic strategies for diabetes and age-related diseases. Furthermore, cysteine-derived MRPs have demonstrated tyrosinase inhibition activity, indicating potential applications in cosmetic formulations and therapeutic interventions for hyperpigmentation disorders [104].
Cysteine resolves its apparent paradox in Maillard chemistry through strategic pathway diversification rather than reaction suppression. The sulfhydryl group acts as a molecular switch, redirecting reaction flux from polymerization-driven browning toward nucleophilic addition compounds and sulfur-containing flavor molecules. This mechanistic understanding enables precise control over Maillard reaction outcomes, allowing researchers to decouple desirable flavor and functionality from undesirable browning and harmful byproducts. The cysteine case study provides a template for developing amino acid-specific Maillard modulation strategies across food, pharmaceutical, and health science applications.
Non-enzymatic browning reactions significantly impact the sensory properties, nutritional value, and safety of thermally processed foods and biological systems [11] [1]. Among these reactions, the Maillard reaction and caramelization represent distinct chemical pathways that often occur simultaneously in complex matrices, presenting significant challenges for researchers seeking to understand, control, or quantify their individual contributions [1] [105]. While both processes generate brown pigments and flavor compounds, they differ fundamentally in their underlying mechanisms, reactant requirements, and product profiles [11] [106].
The Maillard reaction involves amino compounds reacting with carbonyl groups, primarily from reducing sugars [11] [28]. In contrast, caramelization is pyrolytic sugar degradation that occurs without amino compound participation [106] [107]. In real-world systems such as foods, pharmaceutical formulations, and biological models, these pathways frequently intersect and interact, creating complex reaction networks that complicate precise differentiation [1] [105]. This technical guide provides researchers with advanced methodologies to dissect these intertwined pathways through mechanistic understanding, analytical approaches, and experimental design tailored for complex matrices.
Fundamental Chemical Mechanisms
Maillard Reaction Pathway
The Maillard reaction proceeds through three well-defined stages encompassing numerous complex chemical transformations [11] [28]. The initial stage begins with a nucleophilic addition between a carbonyl group (typically from a reducing sugar) and a free amino group (from amino acids, peptides, or proteins) to form a glycosylamine [11] [2]. This intermediate undergoes Amadori rearrangement to form stable 1-amino-1-deoxyketose derivatives (or Heyns products from ketoses) [1]. These early stage reactions are reversible and do not produce color [1].
The intermediate stage involves multiple parallel reaction pathways where Amadori/Heyns products undergo dehydration, fragmentation, and rearrangement, leading to the formation of reactive dicarbonyl compounds [11] [1]. A critical step in this stage is Strecker degradation, where α-dicarbonyls react with amino acids to produce aldehydes (Strecker aldehydes) and α-aminocarbonyls, generating important aroma compounds [11] [1]. Additionally, this stage produces key flavor compounds including furans, pyrazines, and sulfur-containing heterocycles, as well as potentially harmful compounds like acrylamide [11] [1].
The final stage involves polymerization of reactive intermediates into brown, high-molecular-weight nitrogenous polymers called melanoidins [11] [1]. These pigments contribute to the characteristic brown color of Maillard-reacted products and exhibit antioxidant properties [11] [2]. The reaction rate and pathway are highly dependent on pH, temperature, water activity, and the specific reactants present [11] [2].
Figure 1: The three-stage Maillard reaction pathway, illustrating the progression from initial sugar-amine condensation to final melanoidin polymer formation.
Caramelization Pathway
Caramelization is a pyrolytic process of sugar degradation that occurs when carbohydrates are heated above their melting points, typically at high temperatures (≥120°C) [106] [107]. Unlike the Maillard reaction, caramelization proceeds without the involvement of amino compounds and is therefore strictly a sugar decomposition pathway [106]. The process begins with the melting of sugar molecules, followed by elimination of water through condensation reactions [106] [107].
The reaction mechanism involves isomerization, dehydration, and fragmentation steps that generate a complex mixture of products [106]. Key chemical transformations include 1,2-enolization, dehydration reactions that remove water molecules, and fragmentation that breaks sugar carbon chains [106] [107]. These steps produce reactive intermediates including osuloses (α-dicarbonyl compounds) and other fragmentation products that serve as precursors for aroma compounds [107].
The final stage involves polymerization of dehydrated sugar fragments into high-molecular-weight colored polymers collectively known as caramel [106] [107]. Three primary polymer classes have been identified: caramelans (C₂₄H₃₆O₁₈), caramelens (C₃₆H₅₀O₂₅), and caramelins (C₁₂₅H₁₈₈O₈₀) [106]. The process also generates characteristic aroma compounds including diacetyl (buttery), furans (sweet, nutty), and maltol (caramellic) [106] [107].
Figure 2: Caramelization reaction pathway showing sugar degradation and polymerization steps without amino compound involvement.
Comparative Analysis of Browning Pathways
Table 1: Fundamental characteristics differentiating Maillard reaction and caramelization
| Parameter | Maillard Reaction | Caramelization |
|---|---|---|
| Reactants | Reducing sugars + Amino compounds (amino acids, proteins, amines) [11] [28] | Carbohydrates only (sugars, polysaccharides) [106] [107] |
| Nitrogen Content | Essential (incorporated into products) [11] | Absent (no nitrogen in products) [106] |
| Initial Step | Nucleophilic addition followed by Amadori rearrangement [11] [2] | Sugar melting, inversion, and isomerization [106] [107] |
| Key Intermediates | Schiff bases, Amadori products, Strecker aldehydes, α-dicarbonyls [11] [1] | Anhydro sugars, osuloses, fragmentation products [106] [107] |
| Characteristic Products | Melanoidins (nitrogenous brown polymers), pyrazines, pyrroles, heterocyclic nitrogen compounds [11] [1] | Caramelans, caramelens, caramelins (carbonaceous polymers), furans, maltol [106] [107] |
| Temperature Initiation | Can occur at room temperature (slowly), accelerated from 50°C upward [11] | Specific to sugar type: 105°C (fructose) to 180°C (maltose) [106] |
| pH Influence | Rate increases with pH (alkaline conditions) [11] [2] | Rate lowest at neutral pH, accelerated at both acidic and basic conditions [106] |
| Water Activity | Maximum at aw 0.6-0.7 [28] | Proceeds more readily at low aw [107] |
Reactant Specificity and Crossover Pathways
In complex matrices, the distinction between pathways becomes blurred through shared intermediates and parallel reactions [1] [105]. Both pathways generate reactive α-dicarbonyl compounds that can participate in subsequent reactions, creating intersection points between the two processes [11] [1]. For instance, furan formation can occur through both Maillard (via sugar fragmentation) and caramelization pathways [1] [107]. Similarly, HMF (5-hydroxymethylfurfural) can be generated through Maillard reaction (via 1,2-enolization of Amadori products) or caramelization (direct sugar dehydration) [1] [105].
The presence of amino compounds can redirect caramelization pathways toward Maillard-type reactions when reactive carbonyl intermediates are generated [1]. This crossover effect complicates the attribution of specific products to a single pathway in systems containing both sugars and amino compounds [105]. Understanding these intersection points is crucial for designing experiments that can differentiate the contributions of each pathway.
Analytical Strategies for Pathway Differentiation
Chemical Marker Analysis
Table 2: Characteristic markers for distinguishing Maillard and caramelization pathways
| Marker Category | Maillard-Specific Markers | Caramelization-Specific Markers | Shared Markers |
|---|---|---|---|
| Early-Stage Indicators | Furosine (from lysine blockage) [1], Carboxymethyllysine (CML) [1] | Sugar degradation profiles [106] | - |
| Intermediate Compounds | Pyrazines, Pyrroles, Strecker aldehydes [11] [1] | Caramelens, Dihydromaltol [106] [107] | Furans (HMF, furfural) [1] [105] |
| Polymeric Products | Melanoidins (N-containing, 1-10 kDa) [11] [1] | Caramelins (N-free, high MW) [106] | - |
| Elemental Composition | N/C ratio > 0.01 [11] | N/C ratio ≈ 0 [106] | - |
| Spectroscopic Features | Fluorescence at 420-460 nm [1] | Specific caramel polymer UV profiles [106] | Browning at 420 nm [1] [6] |
Advanced Analytical Techniques
Spectroscopic methods provide rapid, non-destructive monitoring of browning progression [1] [13]. UV-Vis spectroscopy tracks browning intensity at 294 nm (intermediate MRPs) and 420 nm (advanced browning) [1] [6]. Fluorescence spectroscopy detects fluorescent MRPs with excitation at 347 nm and emission at 415 nm [1]. FTIR spectroscopy identifies functional group changes during browning reactions [13].
Chromatographic techniques enable precise quantification of specific markers [1] [13]. HPLC and LC-MS/MS separate and quantify non-volatile markers including HMF, furosine, carboxymethyllysine, and Amadori products [1]. GC-MS analyzes volatile compound profiles characteristic of each pathway (pyrazines for Maillard; diacetyl, maltol for caramelization) [1] [13].
Ultrahigh-resolution mass spectrometry (FT-ICR-MS) provides unparalleled insight into the molecular diversity of browning products by delivering exact molecular formulae for hundreds to thousands of compounds simultaneously [6]. This technique has revealed more than 1400 distinct molecular formulae in ribose-amino acid model systems, enabling comprehensive mapping of reaction pathways [6].
Colorimetric measurements in CIELAB color space offer practical, non-destructive assessment of browning progression that correlates with chemical markers [105]. Parameters L* (lightness), a* (red-green), and b* (yellow-blue) track visual browning development, with decreasing L* and increasing a* values indicating advanced browning [105].
Experimental Design for Pathway Elucidation
Model System Approaches
Controlled model systems provide the foundation for understanding fundamental pathways before addressing complex real-world matrices [6]. Binary model systems (single sugar + single amino acid) allow precise manipulation of reaction variables and unambiguous product identification [6]. For example, ribose-glycine systems have revealed more than 300 distinct molecular entities via FT-ICR-MS analysis [6].
Reactant selection critically influences pathway dominance. Studies comparing amino acid reactivity found the order: lysine > cysteine > isoleucine ≈ glycine in ribose systems, with lysine producing >700 MRPs after 10h at 100°C [6]. Sugar reactivity follows: pentoses > hexoses > disaccharides, with ribose demonstrating particularly high reactivity [6].
Selective inhibition strategies enable pathway discrimination. Sulfites inhibit Maillard reactions by carbonyl binding but minimally affect caramelization [1]. Amino acid removal (via ion-exchange resins) or substitution with non-reactive analogs suppresses Maillard pathways while permitting caramelization [105].
Protocol: Differentiating Pathway Contributions in Complex Matrices
Materials and Reagents
- Test matrix (food material, biological sample, or synthetic mixture)
- Internal standards: ¹³C-labeled amino acids, deuterated HMF, stable isotope-labeled markers
- Solvents: HPLC-grade water, acetonitrile, methanol
- Derivatization reagents: O-phenylenediamine (for α-dicarbonyls), oximation reagents
- SPE cartridges: C18, mixed-mode cation-exchange, graphitized carbon
Sample Preparation and Fractionation
- Lyophilize samples and grind to homogeneous powder
- Extract soluble components using 80:20 ethanol:water (v/v) with sonication
- Fractionate using SPE to separate:
- Neutral/acidic fraction (caramelization products)
- Basic fraction (Maillard products)
- Derivatize aliquots for specific marker analysis (e.g., oximation for dicarbonyls)
Parallel Analysis Approach
- Nitrogen elemental analysis of fractions to determine N/C ratios
- LC-MS/MS quantification of pathway-specific markers (Table 2)
- GC-MS profiling of volatile signatures
- FTIR spectroscopy for functional group analysis
Data Interpretation
- Calculate Maillard contribution index (MCI) based on nitrogen-containing marker concentrations
- Determine caramelization contribution index (CCI) from sugar-derived polymer profiles
- Apply multivariate statistical analysis (PCA, PLS-DA) to classify samples by dominant pathway
Figure 3: Experimental workflow for differentiating Maillard and caramelization pathway contributions in complex matrices.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential reagents and materials for browning pathway research
| Category | Reagent/Material | Specifications | Research Application |
|---|---|---|---|
| Sugar Standards | D-Glucose, D-Fructose, D-Ribose, Maltose, Sucrose | ≥99.5% purity, isotopic standards (¹³C-labeled) | Reactant specificity studies, kinetic modeling, internal standards [6] |
| Amino Acid Reagents | L-Lysine, L-Cysteine, L-Glycine, L-Asparagine | ≥98.5% purity, side-chain protected derivatives | Reactant reactivity studies, pathway inhibition, model systems [6] |
| Analytical Standards | 5-HMF, Furfural, N-ε-Carboxymethyllysine, Furosine, Acrylamide | Certified reference materials, isotope-labeled analogs | Quantification calibration, method validation, recovery studies [1] [105] |
| Chromatography | C18 columns (1.8-5μm), HILIC columns, Graphitized carbon SPE | UHPLC-compatible, high efficiency | Separation of polar MRPs, caramelization products, sample cleanup [1] [13] |
| Derivatization Reagents | O-Phenylenediamine, O-(2,3,4,5,6-Pentafluorobenzyl)hydroxylamine | ≥98% purity, MS-compatible | α-Dicarbonyl trapping, carbonyl compound analysis, volatility enhancement [13] |
| Spectroscopic Standards | Melanoidin extracts, Caramel polymer fractions | Characterized molecular weight fractions | Method development, quantitative calibration, comparative studies [1] [106] |
The interplay between Maillard and caramelization pathways in complex matrices represents a significant challenge that requires sophisticated experimental design and analytical approaches. Through the strategic application of chemical marker analysis, advanced separation techniques, and carefully controlled model systems, researchers can dissect the relative contributions of these intertwined pathways. The methodologies outlined in this guide provide a framework for pathway differentiation that spans from fundamental chemical principles to practical experimental protocols.
Future research directions should focus on developing more specific inhibitors for selective pathway suppression, expanding libraries of isotope-labeled standards for precise quantification, and applying computational modeling to predict pathway dominance under specific conditions. As analytical technologies continue to advance, particularly in mass spectrometry and spectroscopic techniques, our ability to unravel the complexity of non-enzymatic browning in real-world systems will correspondingly improve, enabling enhanced control over these chemically and biologically significant reactions.
The Maillard reaction, first described in 1912, represents a fundamental chemical process between reducing sugars and amino compounds that occurs in both food systems and living organisms [108] [55]. This non-enzymatic browning reaction generates advanced glycation end products (AGEs), which have transitioned from being merely chemical curiosities to critical biomarkers and pathogenic mediators in clinical medicine. While in vitro studies have meticulously detailed the complex pathways of AGE formation—beginning with Schiff base formation and progressing through Amadori products to stable end products—the clinical validation of these findings requires demonstration of parallel processes in human patients [6] [109]. This whitepaper examines the critical correlation between in vitro Maillard reaction research and clinical measurements of AGEs, with particular emphasis on HbA1c and other AGE biomarkers, providing researchers and drug development professionals with methodological frameworks for translating experimental findings into clinically relevant applications.
The biochemical pathway of AGE formation follows a consistent pattern across experimental and physiological contexts, beginning with the condensation of reducing sugars with free amino groups of proteins to form Schiff bases, which then undergo Amadori rearrangement to form more stable early glycation products [109] [110]. These compounds subsequently undergo further dehydration, oxidation, and rearrangement reactions to form irreversible AGEs, both fluorescent and non-fluorescent, that accumulate in tissues and contribute to pathological processes through protein cross-linking and receptor-mediated signaling [109] [110]. The following diagram illustrates this core pathway and its clinical connections:
AGE Formation Pathways: From In Vitro Models to Physiological Systems
Fundamental Chemistry of the Maillard Reaction
The Maillard reaction encompasses a complex network of chemical transformations that begin with the nucleophilic addition of amino groups to carbonyl groups of reducing sugars, forming N-substituted glycosylamines that rapidly dehydrate and rearrange into ketosamines known as Amadori products [6] [55]. Under physiological conditions, these early stage reactions are relatively reversible, but prolonged exposure to reducing sugars drives the formation of more stable advanced glycation end products through oxidative and non-oxidative pathways. In vitro studies using model systems, such as ribose-amino acid reactions monitored by Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS), have revealed extraordinary chemodiversity in Maillard reaction products (MRPs), with more than 1,400 distinct molecular formulae identified in simple binary systems [6]. The reactivity and specific pathways are strongly influenced by the nature of the amino acid side chains, with lysine demonstrating the highest reactivity followed by cysteine, isoleucine, and glycine [6].
Structural Diversity of AGEs and Their Pathological Significance
The structural heterogeneity of AGEs presents both challenges and opportunities for clinical correlation. Major AGEs characterized in both experimental models and human subjects include:
Carboxymethyl-lysine (CML) and Carboxyethyl-lysine (CEL): Non-fluorescent, non-crosslinking AGEs that serve as prominent biomarkers of oxidative stress and glycoxidation [109] [110]. These compounds are formed through the oxidative cleavage of Amadori products or through reactions with reactive dicarbonyl compounds like glyoxal and methylglyoxal.
Pentosidine: A fluorescent cross-linking compound that serves as a marker of protein damage and accumulates in long-lived proteins such as collagen [109] [55]. Its fluorescent properties enable detection through specialized spectroscopic methods.
Methylglyoxal-derived hydroimidazolone (MG-H1): The most abundant AGE in physiological systems, formed by the reaction of methylglyoxal with arginine residues [111]. Methylglyoxal originates primarily from glucose degradation and lipid peroxidation pathways.
HbA1c: An Amadori product representing glycation of the N-terminal valine of hemoglobin β-chains, serving as the clinical gold standard for intermediate-term glycemic monitoring [109] [111].
The diversity of these compounds reflects the complexity of Maillard reaction pathways and necessitates multiple analytical approaches for comprehensive clinical assessment.
Clinical Measurement Techniques for AGEs: Methodologies and Applications
Established and Emerging Analytical Platforms
Clinical validation of in vitro findings requires robust analytical methods capable of detecting and quantifying AGEs in complex biological matrices. The following table summarizes the primary techniques currently employed in research and clinical settings:
Table 1: Analytical Methods for AGE Measurement in Clinical Research
| Method | Target Analytes | Sample Types | Key Advantages | Limitations |
|---|---|---|---|---|
| HbA1c Immunoassays [109] | HbA1c | Whole blood | Standardized, automated, clinical gold standard | Limited to hemoglobin, reflects intermediate-term control only |
| Liquid Chromatography-Mass Spectrometry (LC-MS/MS) [109] [110] | CML, CEL, pentosidine, MG-H1 | Serum, plasma, tissue | High specificity and sensitivity, multiplexing capability | Complex instrumentation, requires technical expertise |
| Skin Autofluorescence (SAF) [112] [113] | Total fluorescent AGEs | Skin | Non-invasive, rapid, point-of-care potential | Measures only fluorescent AGEs, influenced by skin pigmentation |
| Enzyme-Linked Immunosorbent Assay (ELISA) [109] [110] | CML, CEL, specific AGE epitopes | Serum, plasma, urine | High throughput, relatively simple protocol | Antibody cross-reactivity, limited to known epitopes |
| Gas Chromatography-Mass Spectrometry (GC-MS) [109] | Specific AGE derivatives | Tissue, serum after derivation | High sensitivity for small molecules | Requires chemical derivation, limited application |
Correlative Data Between AGE Measurements and Clinical Parameters
Clinical studies have established significant correlations between various AGE biomarkers and disease parameters, particularly in diabetes and its complications. Recent research demonstrates that skin AGEs measured by autofluorescence correlate strongly with HbA1c (r=0.404, p<0.001) and show elevation in both type 1 and type 2 diabetes patients compared to healthy controls [113]. Furthermore, skin autofluorescence values demonstrate progressive increases from healthy controls (mean age-adjusted reference: 78.6% within normal range) to type 1 diabetes (53.3% within normal range) to type 2 diabetes (47.1% within normal range), indicating the cumulative burden of glycemic stress across these conditions [113].
In a cross-sectional study of 912 type 2 diabetes patients, the composite metric AGEage (AGEs × age/100) showed significant positive association with urinary albumin-to-creatinine ratio (UACR) levels (β=0.154, 95% CI: 0.126-0.306, P<0.001), indicating the value of AGE measurements in predicting diabetic nephropathy [112]. Moderating factors included HbA1c, body mass index (BMI), and the triglyceride glucose-body mass index (TyG-BMI), highlighting the complex interplay between AGE accumulation and metabolic parameters in driving diabetic complications [112].
Table 2: Clinical Correlations Between AGE Measurements and Disease Parameters
| AGE Biomarker | Clinical Correlation | Population | Statistical Significance | Study Reference |
|---|---|---|---|---|
| Skin Autofluorescence | Positive correlation with HbA1c | DM1 and DM2 patients | r=0.404, p<0.001 | [113] |
| AGEage | Association with UACR (nephropathy marker) | 912 T2DM patients | β=0.154, 95% CI: 0.126-0.306, P<0.001 | [112] |
| Skin Autofluorescence | Elevated in DM2 vs. DM1 | DM1 and DM2 patients | p=0.006 | [113] |
| Skin Autofluorescence | Correlation with liver stiffness | Diabetes patients | r=0.356, p<0.001 | [113] |
| Skin Autofluorescence | Correlation with hepatic steatosis | Diabetes patients | r=0.260, p=0.016 | [113] |
Experimental Protocols for AGE Analysis
Protocol: Skin Autofluorescence Measurement for AGE Assessment
The non-invasive assessment of skin AGEs using autofluorescence has emerged as a valuable tool for evaluating long-term cumulative glycation burden. The following protocol outlines the standardized methodology:
Principle: Advanced glycation end products in the skin exhibit natural fluorescence when excited with specific wavelengths of light. The intensity of emitted light is proportional to the concentration of AGEs in the tissue [113].
Equipment Requirements:
- AGE Reader (Diagnoptics, Groningen, Netherlands) or equivalent device
- Standardized calibration materials
- Computer with manufacturer's software for data acquisition and analysis
Procedure:
- Position the patient in a seated posture with the forearm resting comfortably on a stable surface.
- Ensure the measurement site on the volar aspect of the forearm is clean, dry, and free from optical contaminants such as tattoos, scars, or excessive hair.
- Initiate the measurement sequence using the manufacturer's software interface.
- The device will automatically deliver excitation light at a peak wavelength of 370 nm to approximately 0.1 cm² of skin area.
- Emitted light between 420-600 nm is collected by a built-in spectrometer over a 12-second measurement period.
- The autofluorescence value is calculated as the ratio between emitted light and reflected excitation light.
- Perform three independent measurements at adjacent sites and record the mean value.
- Reference values are automatically adjusted for age using built-in algorithms [112] [113].
Quality Control Considerations:
- Maintain consistent ambient light conditions (semi-darkened room)
- Ensure consistent room temperature (20-24°C)
- Verify device calibration according to manufacturer specifications
- For longitudinal studies, measure the same arm at consistent locations
Data Interpretation: Results are typically expressed as arbitrary units (AU) and compared to age-adjusted reference values. Elevated values indicate increased AGE accumulation and correlate with diabetic complications, including nephropathy, retinopathy, and cardiovascular disease [113].
Protocol: LC-MS/MS Analysis of Serum AGEs
Liquid chromatography coupled with tandem mass spectrometry provides the gold standard for specific AGE quantification in biological fluids.
Sample Preparation:
- Add 100 μL of internal standard solution (typically deuterated CML, CEL, or pentosidine) to 500 μL of serum or plasma.
- Precipitate proteins with 1 mL of cold methanol and vortex vigorously for 30 seconds.
- Centrifuge at 14,000 × g for 15 minutes at 4°C.
- Transfer supernatant to a fresh tube and evaporate to dryness under nitrogen stream.
- Reconstitute dried extract in 100 μL of 0.1% formic acid in water [109] [110].
Chromatographic Conditions:
- Column: C18 reversed-phase (2.1 × 100 mm, 1.7 μm particle size)
- Mobile Phase A: 0.1% formic acid in water
- Mobile Phase B: 0.1% formic acid in acetonitrile
- Gradient: 2-30% B over 12 minutes, 30-95% B over 3 minutes, hold at 95% B for 2 minutes
- Flow Rate: 0.3 mL/min
- Column Temperature: 40°C
- Injection Volume: 10 μL
Mass Spectrometric Parameters:
- Ionization Mode: Positive electrospray ionization (ESI+)
- Ion Source Temperature: 150°C
- Desolvation Temperature: 500°C
- Cone Gas Flow: 50 L/hour
- Desolvation Gas Flow: 800 L/hour
- Detection: Multiple reaction monitoring (MRM) with transitions specific to target AGEs [109]
Quantification: Prepare calibration curves using authentic standards spanning expected physiological ranges (typically 0.1-1000 ng/mL). Quantify samples using the internal standard method with peak area ratios.
The AGE-RAGE Signaling Axis: Mechanistic Insights from Bench to Bedside
The pathological significance of AGEs extends beyond their function as biomarkers to active mediators of cellular dysfunction through receptor-mediated signaling. The receptor for advanced glycation end products (RAGE) serves as a pattern recognition receptor that engages multiple ligands, including AGEs, initiating pro-inflammatory signaling cascades [108] [55]. The following diagram illustrates the key signaling pathways and their clinical implications:
Activation of RAGE by AGE binding triggers multiple intracellular signaling pathways, most notably nuclear factor kappa B (NF-κB), which promotes the transcription of pro-inflammatory cytokines and further upregulates RAGE expression itself, creating a positive feedback loop that sustains inflammation [108] [111]. Additional pathways include MAPK signaling, JAK/STAT activation, and increased production of reactive oxygen species through NADPH oxidase, collectively contributing to the chronic inflammation observed in diabetes, neurodegenerative diseases, and other AGE-related pathologies [110] [111].
The soluble forms of RAGE (sRAGE and esRAGE) function as decoy receptors that neutralize AGEs and inhibit RAGE-mediated signaling, making their circulating levels potential biomarkers of AGE activity and therapeutic response [110]. Clinical studies have demonstrated altered sRAGE levels in various pathological conditions, with obese individuals showing significantly lower sRAGE that increases following weight reduction, corresponding to reduced AGE-mediated inflammation [55].
The Scientist's Toolkit: Essential Research Reagents and Methodologies
Table 3: Essential Research Reagents and Materials for AGE Studies
| Category | Specific Reagents/Assays | Research Applications | Technical Considerations |
|---|---|---|---|
| AGE Standards | CML, CEL, pentosidine, MG-H1 standards | Method validation, calibration curves | Source from reputable suppliers; verify purity by LC-MS |
| Antibodies | Anti-CML, anti-CEL, anti-RAGE monoclonal antibodies | ELISA, immunohistochemistry, Western blot | Validate specificity with knockout controls; check cross-reactivity |
| Assay Kits | Commercial AGE ELISA kits, sRAGE assay kits | High-throughput screening, clinical studies | Compare lot-to-lot variability; establish in-house controls |
| Chromatography | C18 columns, UPLC systems, guard columns | LC-MS/MS analysis of AGEs | Use LC-MS grade solvents; optimize mobile phase additives |
| Mass Spectrometry | Triple quadrupole MS, Q-TOF, FT-ICR-MS | Targeted quantification, untargeted discovery | Establish MRM transitions; optimize collision energies |
| Cell Culture Models | RAGE-transfected cells, primary cell cultures | Signaling studies, drug screening | Monitor passage effects; authenticate cell lines regularly |
| Animal Models | Streptozotocin-induced diabetes, db/db mice, RAGE knockouts | Pathogenesis studies, therapeutic testing | Consider sex-specific differences; control diet composition |
The clinical validation of in vitro Maillard reaction findings requires a multifaceted approach that integrates chemical principles with physiological measurements. HbA1c remains the clinical gold standard for intermediate-term glycemic monitoring, but provides an incomplete picture of the total AGE burden [109] [111]. The expanding repertoire of AGE biomarkers, including CML, CEL, pentosidine, and MG-H1, along with emerging methodologies like skin autofluorescence, provide complementary insights into cumulative glycation stress and its pathological consequences [112] [113].
Future directions in the field include the development of standardized reference materials for AGE quantification, validation of point-of-care devices for rapid AGE assessment, and exploration of therapeutic interventions targeting AGE formation or RAGE signaling [55] [110]. For researchers and drug development professionals, a comprehensive approach that combines multiple analytical platforms—from basic immunoassays to advanced mass spectrometry—will yield the most clinically relevant correlations and accelerate the translation of Maillard reaction research into improved patient outcomes.
Conclusion
The chemistry of non-enzymatic browning, particularly the Maillard reaction, represents a vast and intricate network of pathways with profound implications far beyond food science. For biomedical researchers and drug developers, a deep understanding of these reactions is crucial. The foundational mechanisms explain the formation of AGEs in vivo, linking them directly to the pathophysiology of diabetes, aging, and other chronic diseases. The methodological advances, especially high-resolution mass spectrometry, now allow for an unprecedented mapping of the reaction landscape, revealing both common and amino acid-specific pathways. The ability to troubleshoot and optimize reaction conditions provides a toolkit for potentially mitigating harmful compound formation in both processed foods and biological systems. Future research must focus on further elucidating the structure and biological activity of specific AGEs, developing targeted inhibitors for pathological glycation in humans, and applying this knowledge to improve the stability and efficacy of protein-based therapeutics. The cross-pollination of knowledge between food chemistry and medicine continues to be a fertile ground for groundbreaking discoveries.
References
- 1. Influence of Nonenzymatic Browning Reactions on the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12476023/]
- 2. Maillard Reaction: Mechanism, Influencing Parameters ... [https://www.mdpi.com/2304-8158/14/11/1881]
- 3. Reaction pathways and factors influencing nonenzymatic ... [https://pubmed.ncbi.nlm.nih.gov/34596322/]
- 4. Mechanisms non-enzymatic browning in orange juice ... [https://www.sciencedirect.com/science/article/abs/pii/S0308814619305266]
- 5. The effects of reaction parameters on the non-enzymatic b... [https://www.degruyterbrill.com/document/doi/10.1515/ijfe-2019-0189/html?lang=en&srsltid=AfmBOoo9z93ckUHlNPUE_Fv9x1LQOwfYGUFvVQegCnGL859yz-5eBnBI]
- 6. Insights into the Chemistry of Non-Enzymatic Browning ... [https://www.nature.com/articles/s41598-018-34335-5]
- 7. Reaction kinetics and shelf-life prediction of Maillard ... [https://www.sciencedirect.com/science/article/abs/pii/S0958694623001401]
- 8. Inhibition of polyphenols on Maillard reaction products and ... [https://www.sciencedirect.com/science/article/abs/pii/S094471132400254X]
- 9. Antioxidant packaging films: application for sustainable ... [https://pubmed.ncbi.nlm.nih.gov/41140572/]
- 10. Maillard reaction [https://en.wikipedia.org/wiki/Maillard_reaction]
- 11. Maillard Reaction: Mechanism, Influencing Parameters ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12154226/]
- 12. Maillard reaction product and its complexation with ... [https://www.sciencedirect.com/science/article/abs/pii/S2589014X21001572]
- 13. Research progress of the Maillard reaction process ... [https://www.sciencedirect.com/science/article/abs/pii/S1001841725009210]
- 14. The Maillard reaction and Amadori rearrangement [https://www.biosyn.com/tew/The-Maillard-reaction-and-Amadori-rearrangement.aspx]
- 15. Formation of reactive intermediates from Amadori compounds ... [https://pubmed.ncbi.nlm.nih.gov/7840665/]
- 16. Nucleophilic Addition To Carbonyls [https://www.masterorganicchemistry.com/2022/09/09/nucleophilic-addition/]
- 17. Nucleophilic Addition reactions [https://byjus.com/chemistry/nucleophilic-addition-reactions/]
- 18. Schiff base [https://en.wikipedia.org/wiki/Schiff_base]
- 19. Inhibitory Effect of Maillard Reaction Products on Growth ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC165203/]
- 20. Antioxidant and antibrowning properties of Maillard ... [https://www.sciencedirect.com/science/article/abs/pii/S0083672924000013]
- 21. Strecker Degradation - an overview [https://www.sciencedirect.com/topics/chemistry/strecker-degradation]
- 22. Advancements and obstacles in acrylamide detection and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12173749/]
- 23. Analysis of α-dicarbonyl compounds and volatiles formed ... [https://www.nature.com/articles/s41598-019-41824-8]
- 24. Dicarbonyl-mediated co-formation of aroma compounds ... [https://www.sciencedirect.com/science/article/abs/pii/S0308814625039937]
- 25. Melanoidin and the Maillard Reaction: A Scientific Overview [https://coffeefanatics.jp/en/maillard-reaction-strecker-degradation-a-scientific-overview/]
- 26. Factors That Affect the Accumulation of Strecker Aldehydes in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9146978/]
- 27. Traditional food processing and Acrylamide formation [https://www.sciencedirect.com/science/article/pii/S2405844024062893]
- 28. Maillard Reaction - an overview [https://www.sciencedirect.com/topics/food-science/maillard-reaction]
- 29. Antibacterial properties of Maillard reaction products [https://www.spandidos-publications.com/10.3892/br.2025.2019]
- 30. Food Processing and Maillard Reaction Products: Effect on ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4745522/]
- 31. Maillard Reaction - an overview | ScienceDirect Topics [https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/maillard-reaction]
- 32. Formation of cysteine-S-conjugates in the Maillard reaction ... [https://www.sciencedirect.com/science/article/abs/pii/S0308814613004895]
- 33. Inhibition Mechanism of L-Cysteine on Maillard Reaction ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8234683/]
- 34. Maillard Reaction Chemistry and Applications [https://www.nature.com/research-intelligence/nri-topic-summaries/maillard-reaction-chemistry-and-applications-micro-118285]
- 35. Maillard reactions of ribose 5-phosphate and amino acids [https://pubmed.ncbi.nlm.nih.gov/16037226/]
- 36. Color Development Characteristic and Kinetic Modeling of ... [https://www.mdpi.com/2304-8158/14/12/2136]
- 37. Review Maillard reaction chemistry in formation of critical ... [https://www.sciencedirect.com/science/article/abs/pii/S0308814622013784]
- 38. Insights into flavor and key influencing factors of Maillard ... [https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2022.973677/full]
- 39. A Perspective on the Maillard Reaction and the Analysis of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2642649/]
- 40. Characterization of different stages of Maillard reaction in soy [https://pubs.rsc.org/en/content/articlelanding/2025/fo/d4fo04400b]
- 41. Evolution of Complex Maillard Chemical Reactions ... [https://www.nature.com/articles/s41598-017-03691-z]
- 42. A molecular-level analysis of the beer's Maillard reaction ... [https://www.sciencedirect.com/science/article/abs/pii/S0308814621011183]
- 43. FT-ICR-MS reveals the molecular imprints of the brewing ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10557258/]
- 44. Dietary Melanoidins as Emerging Functional Components [https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2025.1672681/full]
- 45. Maillard Reaction-Derived Carbon Nanodots: Food-Origin ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12389037/]
- 46. Maillard Reaction Explained: What Is the Maillard Reaction? [https://www.masterclass.com/articles/maillard-reaction-explained]
- 47. Maillard reaction in different food products: Effect on ... [https://www.sciencedirect.com/science/article/abs/pii/S0956713523003110]
- 48. Effects of Maillard reaction and its product AGEs on aging ... [https://www.sciencedirect.com/science/article/pii/S2213453024000028]
- 49. Unraveling the development of Maillard reaction indicators ... [https://www.sciencedirect.com/science/article/abs/pii/S0308814625009562]
- 50. Rapid and high-throughput analysis of 5 ... [https://www.nature.com/articles/s41538-025-00431-w]
- 51. Effect of processing conditions and exposure assessment ... [https://www.sciencedirect.com/science/article/abs/pii/S0956713525001082]
- 52. Occurrence and risk assessment of hydroxymethylfurfural ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12084364/]
- 53. An overview on glycation: molecular mechanisms, impact ... [https://link.springer.com/article/10.1007/s12551-024-01188-4]
- 54. Protein glycation in diabetes mellitus [https://www.sciencedirect.com/science/article/abs/pii/S0065242322000993]
- 55. The Development of Maillard Reaction, and Advanced ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7729569/]
- 56. Protein glycation – biomarkers of metabolic dysfunction and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8113047/]
- 57. Advanced Glycation End Products in the Skin: Molecular ... [https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2022.837222/full]
- 58. Sex-specific differences of advanced glycation end products in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12167377/]
- 59. Inhibitory effect of cyclodextrin on Maillard reaction and its ... [https://www.sciencedirect.com/science/article/abs/pii/S0378517323007925]
- 60. Real Time Monitoring the Maillard Reaction Intermediates ... [https://www.longdom.org/open-access/real-time-monitoring-the-maillard-reaction-intermediates-by-hplc-ftir-13676.html]
- 61. How sugars protect proteins in the solid state and during ... [https://www.sciencedirect.com/science/article/pii/S0939641116306956]
- 62. Antioxidant and antibrowning properties of Maillard ... [https://pubmed.ncbi.nlm.nih.gov/38997170/]
- 63. Antioxidant activity of water-soluble Maillard reaction ... [https://www.sciencedirect.com/science/article/abs/pii/S0308814604007447]
- 64. The Interplay between Endogenous and Foodborne Pro ... [https://www.mdpi.com/1422-0067/25/14/7827]
- 65. Antioxidant and Functional Activities of MRPs Derived from ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8615102/]
- 66. Characterization of different stages of Maillard reaction in soy [https://pubs.rsc.org/en/content/articlehtml/2025/fo/d4fo04400b]
- 67. Effect of Temperature and Water Activity on the Quality ... [https://link.springer.com/article/10.1007/s11947-025-03931-5]
- 68. Multiple-factor mathematical modeling of glycine-glucose ... [https://www.sciencedirect.com/science/article/abs/pii/S026087741930473X]
- 69. The effects of reaction parameters on the non-enzymatic b... [https://www.degruyterbrill.com/document/doi/10.1515/ijfe-2019-0189/html?lang=en&srsltid=AfmBOopE08ubcDihJxGrGfaQO-pZxnayXubGVMnvLDTzMOAEY965JNrP]
- 70. Integrative transcriptomics and metabolomics analyses ... [https://bmcplantbiol.biomedcentral.com/articles/10.1186/s12870-025-07150-0]
- 71. Effect of pH on non-enzymatic browning reaction during γ ... [https://www.sciencedirect.com/science/article/abs/pii/S0308814604008684]
- 72. Modeling and Experimental Analysis of Tofu-Drying Kinetics [https://www.mdpi.com/2076-3417/15/19/10319]
- 73. A review of Maillard reaction in food and implications to ... [https://www.sciencedirect.com/science/article/abs/pii/S092422440100022X]
- 74. Maillard Reaction in Flour Product Processing [https://pmc.ncbi.nlm.nih.gov/articles/PMC12345924/]
- 75. Optimization of Maillard Reaction between Glucosamine ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5642803/]
- 76. Non-enzymatic browning and the kinetic model of 5- ... [https://www.sciencedirect.com/science/article/abs/pii/S0926669019302845]
- 77. A systematic review on the determination and analytical ... [https://link.springer.com/article/10.1007/s13197-025-06419-4]
- 78. Nonenzymatic browning reaction of essential amino acids [https://pubmed.ncbi.nlm.nih.gov/10552453/]
- 79. Amino acid - Building Blocks, Structure, Functions [https://www.britannica.com/science/amino-acid/Standard-amino-acids]
- 80. Amino acid - Reactions, Structure, Synthesis [https://www.britannica.com/science/amino-acid/Amino-acid-reactions]
- 81. Biochemistry, Essential Amino Acids - StatPearls - NCBI - NIH [https://www.ncbi.nlm.nih.gov/books/NBK557845/]
- 82. Advanced Glycation End Products in Disease Development ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12024296/]
- 83. Formulation and Processing Strategies to Reduce Acrylamide ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10341540/]
- 84. Acrylamide in food: What it is & How to reduce levels [https://www.eufic.org/en/food-safety/article/acrylamide-qa]
- 85. Acrylamide, a toxic maillard by-product and its inhibition by ... [https://www.frontiersin.org/journals/food-science-and-technology/articles/10.3389/frfst.2022.1072675/full]
- 86. Natural Compounds as Inhibitors of Advanced Glycation ... [https://jyoungpharm.org/10.5530/jyp.20251697]
- 87. 美拉德反应——药物与辅料直接相互作用引起的降解 [https://www.pharnexcloud.com/zixun/sd_4079]
- 88. Influence of pH and Composition on Nonenzymatic ... [https://pubmed.ncbi.nlm.nih.gov/32302128/]
- 89. pH和加热时间对美拉德反应挥发性产物的影响 [https://www.spgykj.com/article/id/SPKJ200904040]
- 90. Maillard Reaction in Flour Product Processing: Mechanism, ... [https://www.mdpi.com/2304-8158/14/15/2721]
- 91. Maillard reaction kinetics in milk powder: Effect of water ... [https://www.sciencedirect.com/science/article/abs/pii/S0958694609001368]
- 92. The Relationship Between Water Activity And The Maillard ... [https://royalcoffee.com/the-relationship-between-water-activity-and-the-maillard-reaction-in-roasting/?srsltid=AfmBOorTo3fawl8pn5fj8jkgYubKky3I9bLJGfFK820Hhd4xW6EGbCMX]
- 93. A study on advanced Maillard reaction in heated casein ... [https://www.sciencedirect.com/science/article/abs/pii/S095869469700071X]
- 94. Development of a novel Maillard reaction-based time ... [https://pubs.rsc.org/en/content/articlehtml/2020/ra/d0ra01440k]
- 95. Comparative effect of different sugars instigating non ... [https://www.redalyc.org/journal/5770/577073671019/html/]
- 96. Protein fructosylation: fructose and the Maillard reaction [https://www.sciencedirect.com/science/article/abs/pii/S0002916523198145]
- 97. Analysis of the browning reaction in a sorbitol/glycine model [https://www.sciencedirect.com/science/article/abs/pii/S0963996923014187]
- 98. Mechanism of high glycine dosage promoting degradation ... [https://www.sciencedirect.com/science/article/abs/pii/S0963996925020800]
- 99. Tracking changes in the optical properties and molecular ... [https://www.sciencedirect.com/science/article/pii/S0043135415301755]
- 100. Maillard Reactions: Nonenzymatic Browning in Food ... [https://www.sciencedirect.com/science/article/pii/S0065262808603481]
- 101. Food Systems and the Environment [https://www.genevaenvironmentnetwork.org/resources/updates/food-systems-and-the-environment/]
- 102. Effect of glycine on reaction of cysteine-xylose: Insights ... [https://www.sciencedirect.com/science/article/abs/pii/S096399691730265X]
- 103. L-cysteine modulates the Maillard reaction [https://www.sciencedirect.com/science/article/abs/pii/S0308814625000998]
- 104. Inhibition effects of Maillard reaction products derived from ... [https://pubs.rsc.org/en/content/articlelanding/2016/ra/c6ra15769f]
- 105. The complex dependence of non-enzymatic browning ... [https://www.sciencedirect.com/science/article/abs/pii/S0023643821007891]
- 106. Caramelization [https://en.wikipedia.org/wiki/Caramelization]
- 107. Caramelization Reaction in Browning [https://kevaflavours.com/caramelization-reaction-in-browning/?srsltid=AfmBOoq1DTp6GvQk5tFiJi8LNJFG1dDtQwTc21BI2CYOnLj_Xsfd8Eoo]
- 108. From Fork to Brain: The Role of AGE–RAGE Signaling and the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12452764/]
- 109. Advanced Glycation End Products (AGEs): Biochemistry ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7104326/]
- 110. Advanced Glycation End Products in Disease ... [https://www.mdpi.com/2076-3921/14/4/492]
- 111. Sex-specific differences of advanced glycation end ... [https://www.nature.com/articles/s41387-025-00379-6]
- 112. The relationship between advanced glycation end products ... [https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2025.1468737/full]
- 113. Skin advanced glycation end-products as indicators of the ... [https://bmcendocrdisord.biomedcentral.com/articles/10.1186/s12902-024-01558-9]